Class 11 Exam > Class 11 Questions > A solution of 500 ml of 0.2 M KOH and 500 ml ...
Start Learning for Free
A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -
- a)T1 = T2
- b)T1 = 2T2
- c)T1 = 4T2
- d)T2 = 9T1
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and...
Let heat evolved in the 1st case is Q1 and that in the 2nd case is Q2. Then Q2 = 1/2Q1
However, Q1 = 1000T1 and Q2 = 500T2
Therefore, 500T2 = ½ 1000T1 i.e. T1 = T2
However, Q1 = 1000T1 and Q2 = 500T2
Therefore, 500T2 = ½ 1000T1 i.e. T1 = T2
Most Upvoted Answer
A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and...
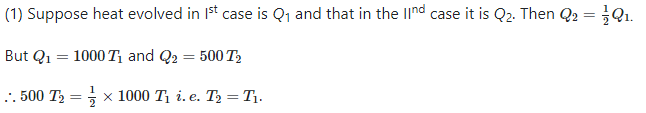
Free Test
FREE
| Start Free Test |
Community Answer
A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and...
Explanation:
When the solution of KOH and HCl is mixed, a neutralization reaction occurs. The reaction between KOH and HCl can be represented as follows:
KOH + HCl → KCl + H2O
The reaction is exothermic, which means it releases heat energy. The rise in temperature observed during the mixing of the solutions is an indication of this heat energy release.
Now let's analyze the given options to determine the relationship between T1 and T2.
a) T1 = T2:
If option a is true, it means that the rise in temperature is the same regardless of the volumes of the solutions used. This would suggest that the heat energy released during the reaction is independent of the amount of reactants used. However, this is not necessarily true. The heat energy released during a reaction is directly proportional to the amount of reactants involved.
b) T1 = 2T2:
If option b is true, it means that the rise in temperature is doubled when the volumes of the solutions are halved. This is not expected as the heat energy released during a reaction is proportional to the amount of reactants involved. Therefore, option b is not true.
c) T1 = 4T2:
If option c is true, it means that the rise in temperature is quadrupled when the volumes of the solutions are halved. Again, this is not expected as the heat energy released during a reaction is proportional to the amount of reactants involved. Therefore, option c is not true.
d) T2 = 9T1:
If option d is true, it means that the rise in temperature is nine times greater when the volumes of the solutions are halved. This is also not expected as the heat energy released during a reaction is proportional to the amount of reactants involved. Therefore, option d is not true.
Therefore, the correct answer is option a: T1 = T2. This means that the rise in temperature observed when the volumes of the solutions are halved is the same as the rise in temperature observed when the volumes are doubled.
When the solution of KOH and HCl is mixed, a neutralization reaction occurs. The reaction between KOH and HCl can be represented as follows:
KOH + HCl → KCl + H2O
The reaction is exothermic, which means it releases heat energy. The rise in temperature observed during the mixing of the solutions is an indication of this heat energy release.
Now let's analyze the given options to determine the relationship between T1 and T2.
a) T1 = T2:
If option a is true, it means that the rise in temperature is the same regardless of the volumes of the solutions used. This would suggest that the heat energy released during the reaction is independent of the amount of reactants used. However, this is not necessarily true. The heat energy released during a reaction is directly proportional to the amount of reactants involved.
b) T1 = 2T2:
If option b is true, it means that the rise in temperature is doubled when the volumes of the solutions are halved. This is not expected as the heat energy released during a reaction is proportional to the amount of reactants involved. Therefore, option b is not true.
c) T1 = 4T2:
If option c is true, it means that the rise in temperature is quadrupled when the volumes of the solutions are halved. Again, this is not expected as the heat energy released during a reaction is proportional to the amount of reactants involved. Therefore, option c is not true.
d) T2 = 9T1:
If option d is true, it means that the rise in temperature is nine times greater when the volumes of the solutions are halved. This is also not expected as the heat energy released during a reaction is proportional to the amount of reactants involved. Therefore, option d is not true.
Therefore, the correct answer is option a: T1 = T2. This means that the rise in temperature observed when the volumes of the solutions are halved is the same as the rise in temperature observed when the volumes are doubled.

|
Explore Courses for Class 11 exam
|

|
Question Description
A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer?.
A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer?.
Solutions for A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer?, a detailed solution for A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A solution of 500 ml of 0.2 M KOH and 500 ml of 0.2 M HCl is mixed and stirred; the rise in temperature is T1. The experiment is repeated using 250 ml of each solution, the temperature raised is T2. Which of the following is true -a)T1= T2b)T1= 2T2c)T1= 4T2d)T2= 9T1Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























