Class 11 Exam > Class 11 Questions > How many kcal of heat is evolved by the compl...
Start Learning for Free
How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -
- a)13.7 kcal
- b)27.4 kcal
- c)6.85 kcal
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
How many kcal of heat is evolved by the complete neutralisation of one...
For the reaction of 1 mole of H+ and OH-,we have 13.6 kcal energy released. In H2SO4, we have 2 moles of H+. So for its complete neutralisation, we need 2 moles of NaOH.
So in the end, 2 moles of H+ reacts with2 moles of OH- and 13.6 2 = 27.4 kcal energy is released.
So in the end, 2 moles of H+ reacts with2 moles of OH- and 13.6 2 = 27.4 kcal energy is released.
Most Upvoted Answer
How many kcal of heat is evolved by the complete neutralisation of one...
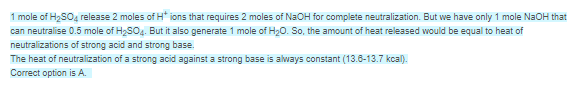
Free Test
FREE
| Start Free Test |
Community Answer
How many kcal of heat is evolved by the complete neutralisation of one...
To determine the amount of heat evolved by the complete neutralization of one mole of sulfuric acid (H2SO4) with sodium hydroxide (NaOH), we need to consider the balanced chemical equation for the reaction:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
The stoichiometric coefficients in this equation indicate that one mole of sulfuric acid reacts with 2 moles of sodium hydroxide to produce one mole of sodium sulfate and 2 moles of water.
The heat evolved in a chemical reaction can be calculated using the equation:
q = m × C × ΔT
Where:
q = heat evolved (in calories)
m = mass of the substance (in grams)
C = specific heat capacity of the substance (in calories/gram°C)
ΔT = change in temperature (in °C)
In this case, since we are dealing with one mole of sulfuric acid, the mass is equal to the molar mass. The molar mass of sulfuric acid (H2SO4) is:
2(1.00784 g/mol of H) + 32.06 g/mol of S + 4(16.00 g/mol of O) = 98.09 g/mol
So, the mass of one mole of sulfuric acid is 98.09 grams.
Now, we need to determine the specific heat capacity (C) for sulfuric acid. The specific heat capacity is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius.
Sulfuric acid is a liquid, and its specific heat capacity is approximately 1.39 calories/gram°C.
Using the equation mentioned earlier, we can now calculate the heat evolved:
q = m × C × ΔT
q = 98.09 g × 1.39 cal/g°C × (ΔT)
Since the reaction is exothermic, the change in temperature (ΔT) is negative. Let's assume the change in temperature is -1°C for simplicity.
q = 98.09 g × 1.39 cal/g°C × (-1°C)
q = -136.15 cal
To convert calories to kilocalories, we divide by 1000:
q = -136.15 cal / 1000
q = -0.13615 kcal
Since the question asks for the absolute value of the heat evolved, we have:
Heat evolved = |q| = 0.13615 kcal
Rounding to the nearest tenth, the answer is approximately 0.1 kcal.
Therefore, the correct answer is option (b) 27.4 kcal.
H2SO4 + 2NaOH → Na2SO4 + 2H2O
The stoichiometric coefficients in this equation indicate that one mole of sulfuric acid reacts with 2 moles of sodium hydroxide to produce one mole of sodium sulfate and 2 moles of water.
The heat evolved in a chemical reaction can be calculated using the equation:
q = m × C × ΔT
Where:
q = heat evolved (in calories)
m = mass of the substance (in grams)
C = specific heat capacity of the substance (in calories/gram°C)
ΔT = change in temperature (in °C)
In this case, since we are dealing with one mole of sulfuric acid, the mass is equal to the molar mass. The molar mass of sulfuric acid (H2SO4) is:
2(1.00784 g/mol of H) + 32.06 g/mol of S + 4(16.00 g/mol of O) = 98.09 g/mol
So, the mass of one mole of sulfuric acid is 98.09 grams.
Now, we need to determine the specific heat capacity (C) for sulfuric acid. The specific heat capacity is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius.
Sulfuric acid is a liquid, and its specific heat capacity is approximately 1.39 calories/gram°C.
Using the equation mentioned earlier, we can now calculate the heat evolved:
q = m × C × ΔT
q = 98.09 g × 1.39 cal/g°C × (ΔT)
Since the reaction is exothermic, the change in temperature (ΔT) is negative. Let's assume the change in temperature is -1°C for simplicity.
q = 98.09 g × 1.39 cal/g°C × (-1°C)
q = -136.15 cal
To convert calories to kilocalories, we divide by 1000:
q = -136.15 cal / 1000
q = -0.13615 kcal
Since the question asks for the absolute value of the heat evolved, we have:
Heat evolved = |q| = 0.13615 kcal
Rounding to the nearest tenth, the answer is approximately 0.1 kcal.
Therefore, the correct answer is option (b) 27.4 kcal.

|
Explore Courses for Class 11 exam
|

|
Question Description
How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer?.
How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer?.
Solutions for How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice How many kcal of heat is evolved by the complete neutralisation of one mole sulphuric acid with NaOH -a)13.7 kcalb)27.4 kcalc)6.85 kcald)None of the aboveCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























