Class 11 Exam > Class 11 Questions > Geometrical isomers can bea)Diastereomers or ...
Start Learning for Free
Geometrical isomers can be
- a)Diastereomers or Enantiomers
- b)Structural isomers
- c)Inter convertable at room temprature.
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural i...
Geometrical Isomers can be Diastereomers or Enantiomers.

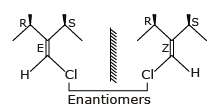
Because geometrical isomers are around restricted rotation which can not be rotate or interconvertable.
Because geometrical isomers are around restricted rotation which can not be rotate or interconvertable.
Most Upvoted Answer
Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural i...
Geometrical isomers are a type of stereoisomers that occur due to restricted rotation around a double bond or a ring structure. They have the same molecular formula and connectivity but differ in the spatial arrangement of atoms in the molecule. Geometrical isomerism is commonly observed in compounds with double bonds, such as alkenes and cyclic compounds.
a) Diastereomers or Enantiomers:
The correct answer is option A - Geometrical isomers can be diastereomers or enantiomers. Let's understand each of these terms:
1. Diastereomers: Diastereomers are stereoisomers that have different spatial arrangements around one or more stereocenters in a molecule. In the case of geometrical isomerism, the double bond or ring structure acts as a stereocenter. Diastereomers have different physical and chemical properties and can be separated using conventional separation techniques. They have different names and exhibit different melting points, boiling points, and optical activities.
2. Enantiomers: Enantiomers are a specific type of stereoisomers that are non-superimposable mirror images of each other. They have the same connectivity and molecular formula but differ in the spatial arrangement of atoms in three-dimensional space. Enantiomers have identical physical and chemical properties except for their interaction with polarized light. They rotate the plane of polarized light in equal magnitudes but in opposite directions. Enantiomers are named using R and S configuration or D and L nomenclature.
b) Structural isomers:
Structural isomers are a type of isomers that have the same molecular formula but differ in the connectivity of atoms within the molecule. In structural isomerism, the atoms are connected in different ways, leading to different chemical and physical properties. Geometrical isomers are not classified as structural isomers because they have the same connectivity of atoms.
c) Interconvertible at room temperature:
Geometrical isomers are not interconvertible at room temperature because they require the breaking and reformation of chemical bonds for interconversion. Interconversion between geometrical isomers typically requires the application of heat or the use of a catalyst.
In conclusion, geometrical isomers can be diastereomers or enantiomers, but they are not classified as structural isomers. They are not interconvertible at room temperature.
a) Diastereomers or Enantiomers:
The correct answer is option A - Geometrical isomers can be diastereomers or enantiomers. Let's understand each of these terms:
1. Diastereomers: Diastereomers are stereoisomers that have different spatial arrangements around one or more stereocenters in a molecule. In the case of geometrical isomerism, the double bond or ring structure acts as a stereocenter. Diastereomers have different physical and chemical properties and can be separated using conventional separation techniques. They have different names and exhibit different melting points, boiling points, and optical activities.
2. Enantiomers: Enantiomers are a specific type of stereoisomers that are non-superimposable mirror images of each other. They have the same connectivity and molecular formula but differ in the spatial arrangement of atoms in three-dimensional space. Enantiomers have identical physical and chemical properties except for their interaction with polarized light. They rotate the plane of polarized light in equal magnitudes but in opposite directions. Enantiomers are named using R and S configuration or D and L nomenclature.
b) Structural isomers:
Structural isomers are a type of isomers that have the same molecular formula but differ in the connectivity of atoms within the molecule. In structural isomerism, the atoms are connected in different ways, leading to different chemical and physical properties. Geometrical isomers are not classified as structural isomers because they have the same connectivity of atoms.
c) Interconvertible at room temperature:
Geometrical isomers are not interconvertible at room temperature because they require the breaking and reformation of chemical bonds for interconversion. Interconversion between geometrical isomers typically requires the application of heat or the use of a catalyst.
In conclusion, geometrical isomers can be diastereomers or enantiomers, but they are not classified as structural isomers. They are not interconvertible at room temperature.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer?
Question Description
Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer?.
Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Geometrical isomers can bea)Diastereomers or Enantiomersb)Structural isomersc)Inter convertable at room temprature.d)None of theseCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























