Class 11 Exam > Class 11 Questions > Bottles containing C6H5I and C6H5CH2I lost th...
Start Learning for Free
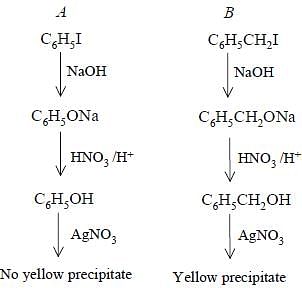
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.
Which one of the following statements is true for this experiment ?
Which one of the following statements is true for this experiment ?
[AIEEE-2003]
- a)B was C6H5I
- b)Addition of HNO3 was unnecessary
- c)A was C6H5IB was C6H5I
- d)A was C6H5CH2I
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Bottles containing C6H5I and C6H5CH2I lost their original labels. They...
Most Upvoted Answer
Bottles containing C6H5I and C6H5CH2I lost their original labels. They...
Benzyl cation is highly stable due to resonance. So when C6H5CH2I will react with AgNO3 to produce pale yellow PPT. of AgI. But as Benzene anion is less stable than benzylic cation it will not give PPT with AgNO3. Hence C6H5CH2I is B and the other is A
Free Test
FREE
| Start Free Test |
Community Answer
Bottles containing C6H5I and C6H5CH2I lost their original labels. They...
Explanation:
The experiment involves boiling the unknown substances with NaOH, acidifying the solution with HNO3, and adding AgNO3 to observe a yellow precipitate.
Step 1: Boiling with NaOH solution
When C6H5I (Phenyl iodide) is boiled with NaOH solution, it undergoes hydrolysis to form sodium phenoxide (C6H5ONa) and NaI. The reaction can be represented as follows:
C6H5I + NaOH → C6H5ONa + NaI
When C6H5CH2I (Benzyl iodide) is boiled with NaOH solution, it also undergoes hydrolysis to form sodium benzyl alcoholate (C6H5CH2ONa) and NaI. The reaction can be represented as follows:
C6H5CH2I + NaOH → C6H5CH2ONa + NaI
Step 2: Acidifying the solution with HNO3
After the hydrolysis step, the resulting solution is made acidic with dilute HNO3. The purpose of this step is to neutralize the excess NaOH and convert the sodium salts back into their respective acids. The reaction can be represented as follows:
C6H5ONa + HNO3 → C6H5OH + NaNO3
C6H5CH2ONa + HNO3 → C6H5CH2OH + NaNO3
Step 3: Adding AgNO3 solution
After acidifying the solution, some AgNO3 solution is added. AgNO3 reacts with the phenols formed in the previous step to form yellow precipitates, which are silver salts of the respective acids. The reaction can be represented as follows:
C6H5OH + AgNO3 → C6H5AgO + HNO3
C6H5CH2OH + AgNO3 → C6H5CH2AgO + HNO3
Observation:
The statement in the question mentions that substance B gave a yellow precipitate. This indicates that substance B is a phenol, as it reacted with AgNO3 to form a silver salt. Therefore, it can be concluded that B was C6H5I.
Conclusion:
By process of elimination, the only option that remains is option C: A was C6H5I. Since B was C6H5I, A must be C6H5CH2I.
The experiment involves boiling the unknown substances with NaOH, acidifying the solution with HNO3, and adding AgNO3 to observe a yellow precipitate.
Step 1: Boiling with NaOH solution
When C6H5I (Phenyl iodide) is boiled with NaOH solution, it undergoes hydrolysis to form sodium phenoxide (C6H5ONa) and NaI. The reaction can be represented as follows:
C6H5I + NaOH → C6H5ONa + NaI
When C6H5CH2I (Benzyl iodide) is boiled with NaOH solution, it also undergoes hydrolysis to form sodium benzyl alcoholate (C6H5CH2ONa) and NaI. The reaction can be represented as follows:
C6H5CH2I + NaOH → C6H5CH2ONa + NaI
Step 2: Acidifying the solution with HNO3
After the hydrolysis step, the resulting solution is made acidic with dilute HNO3. The purpose of this step is to neutralize the excess NaOH and convert the sodium salts back into their respective acids. The reaction can be represented as follows:
C6H5ONa + HNO3 → C6H5OH + NaNO3
C6H5CH2ONa + HNO3 → C6H5CH2OH + NaNO3
Step 3: Adding AgNO3 solution
After acidifying the solution, some AgNO3 solution is added. AgNO3 reacts with the phenols formed in the previous step to form yellow precipitates, which are silver salts of the respective acids. The reaction can be represented as follows:
C6H5OH + AgNO3 → C6H5AgO + HNO3
C6H5CH2OH + AgNO3 → C6H5CH2AgO + HNO3
Observation:
The statement in the question mentions that substance B gave a yellow precipitate. This indicates that substance B is a phenol, as it reacted with AgNO3 to form a silver salt. Therefore, it can be concluded that B was C6H5I.
Conclusion:
By process of elimination, the only option that remains is option C: A was C6H5I. Since B was C6H5I, A must be C6H5CH2I.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer?
Question Description
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer?.
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer?.
Solutions for Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.Which one of the following statements is true for this experiment ? [AIEEE-2003]a)B was C6H5Ib)Addition of HNO3 was unnecessaryc)A was C6H5IB was C6H5Id)A was C6H5CH2ICorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























