Mechanical Engineering Exam > Mechanical Engineering Questions > Dynamic viscosity of most of the gases with r...
Start Learning for Free
Dynamic viscosity of most of the gases with rise in temperature...
- a)Increases
- b)Decreases
- c)Remains unaffected
- d)Unpredictable
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
Dynamic viscosity of most of the gases with rise in temperature...a)In...
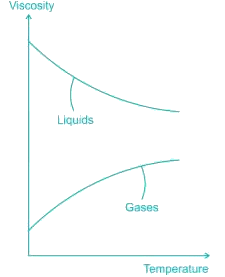
The viscosity of liquids decreases with temperature, whereas the viscosity of gases increases with temperature. This is because in a liquid the molecules possess more energy at higher temperatures, and they can oppose the large cohesive inter-molecular forces more strongly. As a result, the energized liquid molecules can move more freely.
In a gas, on the other hand, the inter-molecular forces are negligible, and the gas molecules at high temperatures move randomly at higher velocities. This results in more molecular collisions per unit volume per unit time and therefore in greater resistance to flow.
Most Upvoted Answer
Dynamic viscosity of most of the gases with rise in temperature...a)In...
Understanding Dynamic Viscosity in Gases
Dynamic viscosity refers to a fluid's internal resistance to flow. In gases, this property is significantly influenced by temperature.
Temperature's Effect on Gas Viscosity
- As temperature increases, the kinetic energy of gas molecules also rises.
- Higher kinetic energy causes gas molecules to move more rapidly and collide with one another more frequently.
Increased Molecular Activity
- With increased molecular motion, the chances of intermolecular collisions grow.
- This results in greater resistance to flow, hence increasing the dynamic viscosity of the gas.
Intermolecular Forces
- Although gases have weaker intermolecular forces compared to liquids, these forces still play a role.
- At elevated temperatures, the energy can overcome some of these forces, but the net effect is a rise in viscosity due to enhanced molecular activity.
Comparison with Liquids
- Unlike gases, the dynamic viscosity of liquids typically decreases with an increase in temperature due to reduced intermolecular attraction.
- This contrast highlights the unique behavior of gases with temperature variations.
Conclusion
- In summary, the correct answer is option 'A': the dynamic viscosity of most gases increases with rising temperature.
- Understanding this relationship is crucial in applications such as fluid dynamics, thermodynamics, and various engineering processes.
Dynamic viscosity refers to a fluid's internal resistance to flow. In gases, this property is significantly influenced by temperature.
Temperature's Effect on Gas Viscosity
- As temperature increases, the kinetic energy of gas molecules also rises.
- Higher kinetic energy causes gas molecules to move more rapidly and collide with one another more frequently.
Increased Molecular Activity
- With increased molecular motion, the chances of intermolecular collisions grow.
- This results in greater resistance to flow, hence increasing the dynamic viscosity of the gas.
Intermolecular Forces
- Although gases have weaker intermolecular forces compared to liquids, these forces still play a role.
- At elevated temperatures, the energy can overcome some of these forces, but the net effect is a rise in viscosity due to enhanced molecular activity.
Comparison with Liquids
- Unlike gases, the dynamic viscosity of liquids typically decreases with an increase in temperature due to reduced intermolecular attraction.
- This contrast highlights the unique behavior of gases with temperature variations.
Conclusion
- In summary, the correct answer is option 'A': the dynamic viscosity of most gases increases with rising temperature.
- Understanding this relationship is crucial in applications such as fluid dynamics, thermodynamics, and various engineering processes.

|
Explore Courses for Mechanical Engineering exam
|

|
Question Description
Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer?.
Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Dynamic viscosity of most of the gases with rise in temperature...a)Increasesb)Decreasesc)Remains unaffectedd)UnpredictableCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















