Chemistry Exam > Chemistry Questions > The fraction of the total current carried by ...
Start Learning for Free
The fraction of the total current carried by an ion is known as:
- a)Transport number of that ion
- b)Conductance of that ion
- c)Both a and b
- d)None of these.
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
The fraction of the total current carried by an ion is known as:a)Tran...
The fraction of the total current carried by each ion is called the Transport number.
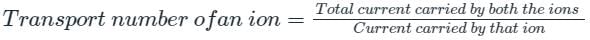
It is also called the transference number or Hittorf number.
Most Upvoted Answer
The fraction of the total current carried by an ion is known as:a)Tran...
Transport number of an ion:
The transport number of an ion refers to the fraction of the total current carried by that specific ion in an electrolytic solution or a cell. It is denoted by the symbol "t" and is expressed as a decimal or fraction.
Conductance of an ion:
Conductance is a measure of an ion's ability to conduct electric current. It represents the ease with which ions move through an electrolyte solution. The conductance of an ion depends on its concentration, mobility, and charge.
Explanation:
In an electrolytic solution or a cell, multiple ions may be present. When an electric current is passed through the solution, the ions move towards the electrodes, carrying the charge. The transport number of an ion indicates the proportion of the total current that is carried by that specific ion.
The transport number of an ion can be determined experimentally using various techniques, such as the Hittorf's method or the moving boundary method. These methods allow for the measurement of changes in the concentrations of ions at the electrodes and help in calculating the transport number.
The conductance of an ion is related to its transport number. The conductance is influenced by factors such as ion concentration, ion mobility, and charge. However, it does not directly represent the fraction of total current carried by an ion.
Conclusion:
In conclusion, the fraction of the total current carried by an ion is known as the transport number of that ion. It is an important parameter in understanding the contribution of individual ions to the overall current flow in an electrolytic solution or a cell. The conductance of an ion, on the other hand, represents its ability to conduct the electric current and is influenced by various factors. Therefore, the correct answer is option 'A' - Transport number of that ion.
The transport number of an ion refers to the fraction of the total current carried by that specific ion in an electrolytic solution or a cell. It is denoted by the symbol "t" and is expressed as a decimal or fraction.
Conductance of an ion:
Conductance is a measure of an ion's ability to conduct electric current. It represents the ease with which ions move through an electrolyte solution. The conductance of an ion depends on its concentration, mobility, and charge.
Explanation:
In an electrolytic solution or a cell, multiple ions may be present. When an electric current is passed through the solution, the ions move towards the electrodes, carrying the charge. The transport number of an ion indicates the proportion of the total current that is carried by that specific ion.
The transport number of an ion can be determined experimentally using various techniques, such as the Hittorf's method or the moving boundary method. These methods allow for the measurement of changes in the concentrations of ions at the electrodes and help in calculating the transport number.
The conductance of an ion is related to its transport number. The conductance is influenced by factors such as ion concentration, ion mobility, and charge. However, it does not directly represent the fraction of total current carried by an ion.
Conclusion:
In conclusion, the fraction of the total current carried by an ion is known as the transport number of that ion. It is an important parameter in understanding the contribution of individual ions to the overall current flow in an electrolytic solution or a cell. The conductance of an ion, on the other hand, represents its ability to conduct the electric current and is influenced by various factors. Therefore, the correct answer is option 'A' - Transport number of that ion.
Free Test
FREE
| Start Free Test |
Community Answer
The fraction of the total current carried by an ion is known as:a)Tran...
Because fraction means 1 m se kitna
and that's a transport number
and that's a transport number

|
Explore Courses for Chemistry exam
|

|
Question Description
The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer?.
The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer?.
Solutions for The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer?, a detailed solution for The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The fraction of the total current carried by an ion is known as:a)Transport number of that ionb)Conductance of that ionc)Both a and bd)None of these.Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















