Class 12 Exam > Class 12 Questions > The effective atomic number of Fe inFe(CO)5is...
Start Learning for Free
The effective atomic number of Fe in Fe(CO)5 is
- a)34
- b)24
- c)36
- d)26
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct a...

Most Upvoted Answer
The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct a...
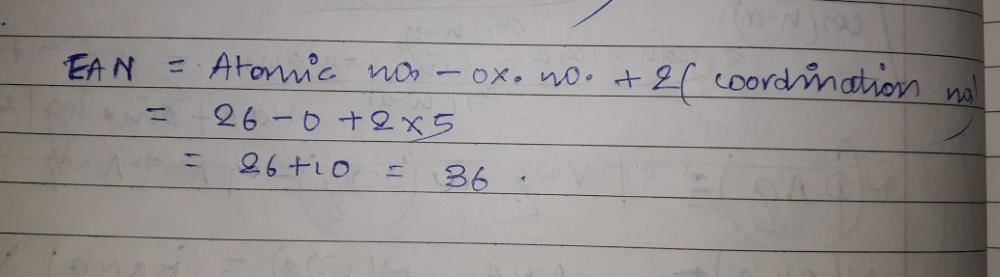
Free Test
FREE
| Start Free Test |
Community Answer
The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct a...
Effective Atomic Number of Fe in Fe(CO)5
Definition of Effective Atomic Number:
The effective atomic number (EAN) is a concept used to describe the electron configuration of transition metal complexes. It assumes that the metal ion in the complex has achieved a noble gas electron configuration by accepting electrons from ligands.
Steps to Find EAN:
1. Identify the oxidation state of the metal ion in the complex.
2. Calculate the total number of electrons donated by the ligands to the metal ion.
3. Calculate the number of electrons the metal ion needs to achieve a noble gas configuration.
4. Calculate the effective atomic number by adding the number of electrons donated by the ligands to the atomic number of the metal ion.
Fe(CO)5:
1. The oxidation state of Fe in Fe(CO)5 is zero.
2. Each CO ligand donates one electron to Fe. Therefore, the total number of electrons donated by the ligands is 5.
3. Fe needs 8 electrons to achieve a noble gas configuration (like the element Ar), since Fe has 26 electrons in its ground state configuration and the Ar configuration has 18 electrons.
4. The effective atomic number of Fe is calculated by adding the number of electrons donated by the ligands to the atomic number of Fe:
EAN = 26 + 5 = 31
However, this value does not match any of the given options. This is because the EAN assumes that the ligands are completely ionic, and this is not the case for CO ligands. CO is a pi-acceptor ligand, which means that it can accept electrons from the metal ion, in addition to donating electrons. Therefore, the EAN is not an accurate representation of the electron configuration of Fe in Fe(CO)5.
Instead, the electron configuration of Fe in Fe(CO)5 is best described by molecular orbital theory. In this theory, the Fe-CO bonds are formed by the overlap of the Fe d orbitals with the CO pi* orbitals. The Fe d orbitals are split into two sets, one of which is lower in energy than the pi* orbitals, and the other of which is higher in energy. The lower set forms sigma bonds with the CO ligands, while the higher set remains unoccupied. The electrons from the CO pi* orbitals fill the unoccupied Fe d orbitals, forming a set of bonding and anti-bonding molecular orbitals.
In summary, the effective atomic number of Fe in Fe(CO)5 is 31, but this value is not an accurate representation of the electron configuration of Fe in the complex. The electron configuration of Fe in Fe(CO)5 is best described by molecular orbital theory.
Definition of Effective Atomic Number:
The effective atomic number (EAN) is a concept used to describe the electron configuration of transition metal complexes. It assumes that the metal ion in the complex has achieved a noble gas electron configuration by accepting electrons from ligands.
Steps to Find EAN:
1. Identify the oxidation state of the metal ion in the complex.
2. Calculate the total number of electrons donated by the ligands to the metal ion.
3. Calculate the number of electrons the metal ion needs to achieve a noble gas configuration.
4. Calculate the effective atomic number by adding the number of electrons donated by the ligands to the atomic number of the metal ion.
Fe(CO)5:
1. The oxidation state of Fe in Fe(CO)5 is zero.
2. Each CO ligand donates one electron to Fe. Therefore, the total number of electrons donated by the ligands is 5.
3. Fe needs 8 electrons to achieve a noble gas configuration (like the element Ar), since Fe has 26 electrons in its ground state configuration and the Ar configuration has 18 electrons.
4. The effective atomic number of Fe is calculated by adding the number of electrons donated by the ligands to the atomic number of Fe:
EAN = 26 + 5 = 31
However, this value does not match any of the given options. This is because the EAN assumes that the ligands are completely ionic, and this is not the case for CO ligands. CO is a pi-acceptor ligand, which means that it can accept electrons from the metal ion, in addition to donating electrons. Therefore, the EAN is not an accurate representation of the electron configuration of Fe in Fe(CO)5.
Instead, the electron configuration of Fe in Fe(CO)5 is best described by molecular orbital theory. In this theory, the Fe-CO bonds are formed by the overlap of the Fe d orbitals with the CO pi* orbitals. The Fe d orbitals are split into two sets, one of which is lower in energy than the pi* orbitals, and the other of which is higher in energy. The lower set forms sigma bonds with the CO ligands, while the higher set remains unoccupied. The electrons from the CO pi* orbitals fill the unoccupied Fe d orbitals, forming a set of bonding and anti-bonding molecular orbitals.
In summary, the effective atomic number of Fe in Fe(CO)5 is 31, but this value is not an accurate representation of the electron configuration of Fe in the complex. The electron configuration of Fe in Fe(CO)5 is best described by molecular orbital theory.

|
Explore Courses for Class 12 exam
|

|
Question Description
The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer?.
The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer?.
Solutions for The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer?, a detailed solution for The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The effective atomic number of Fe inFe(CO)5isa)34b)24c)36d)26Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















