Class 12 Exam > Class 12 Questions > Formic acid is obtained when [1994]a)Calcium ...
Start Learning for Free
Formic acid is obtained when [1994]
- a)Calcium acetate is heated with conc. H2SO4
- b)Calcium formate is heated with calciumacetate
- c)Glycerol is heated with oxalic acid at 373 K
- d)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Formic acid is obtained when [1994]a)Calcium acetate is heated with co...
When glycerol is heated with oxalic acid
following reaction occurs.
following reaction occurs.
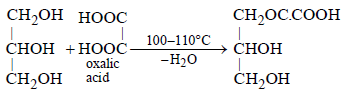
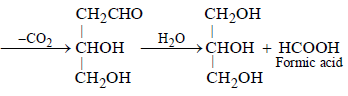
Most Upvoted Answer
Formic acid is obtained when [1994]a)Calcium acetate is heated with co...
To obtain formic acid, glycerol needs to be heated with oxalic acid at 373 K. This reaction is represented by the following equation:
C3H8O3 + H2C2O4 → HCOOH + CO2 + 2H2O
Here is a detailed explanation of why option C is the correct answer:
1. Reaction Overview:
- Glycerol: Glycerol, also known as glycerin, is a colorless and odorless liquid. It is a trihydric alcohol with the chemical formula C3H8O3.
- Oxalic Acid: Oxalic acid is a dicarboxylic acid with the chemical formula H2C2O4. It is a strong acid that occurs naturally in many plants.
- Formic Acid: Formic acid, also known as methanoic acid, is a colorless liquid with a pungent odor. It has the chemical formula HCOOH.
2. Reaction Mechanism:
- The reaction between glycerol and oxalic acid is an esterification reaction, where the hydroxyl groups (-OH) of glycerol react with the carboxyl groups (-COOH) of oxalic acid.
- The reaction is catalyzed by heat, which provides the necessary activation energy for the reaction to occur.
- The esterification reaction results in the formation of formic acid (HCOOH), carbon dioxide (CO2), and water (H2O) as byproducts.
3. Explanation of other Options:
a) Calcium acetate is heated with conc. H2SO4: This reaction does not yield formic acid. Instead, it would result in the formation of acetic acid and calcium sulfate.
b) Calcium formate is heated with calcium acetate: This reaction does not yield formic acid. It would lead to the formation of calcium oxalate and calcium carbonate.
d) Acetaldehyde is oxidized with K2Cr2O7 and H2SO4: This reaction does not yield formic acid. It would result in the formation of acetic acid.
In conclusion, the correct option to obtain formic acid from the given choices is option C, where glycerol is heated with oxalic acid at 373 K.
C3H8O3 + H2C2O4 → HCOOH + CO2 + 2H2O
Here is a detailed explanation of why option C is the correct answer:
1. Reaction Overview:
- Glycerol: Glycerol, also known as glycerin, is a colorless and odorless liquid. It is a trihydric alcohol with the chemical formula C3H8O3.
- Oxalic Acid: Oxalic acid is a dicarboxylic acid with the chemical formula H2C2O4. It is a strong acid that occurs naturally in many plants.
- Formic Acid: Formic acid, also known as methanoic acid, is a colorless liquid with a pungent odor. It has the chemical formula HCOOH.
2. Reaction Mechanism:
- The reaction between glycerol and oxalic acid is an esterification reaction, where the hydroxyl groups (-OH) of glycerol react with the carboxyl groups (-COOH) of oxalic acid.
- The reaction is catalyzed by heat, which provides the necessary activation energy for the reaction to occur.
- The esterification reaction results in the formation of formic acid (HCOOH), carbon dioxide (CO2), and water (H2O) as byproducts.
3. Explanation of other Options:
a) Calcium acetate is heated with conc. H2SO4: This reaction does not yield formic acid. Instead, it would result in the formation of acetic acid and calcium sulfate.
b) Calcium formate is heated with calcium acetate: This reaction does not yield formic acid. It would lead to the formation of calcium oxalate and calcium carbonate.
d) Acetaldehyde is oxidized with K2Cr2O7 and H2SO4: This reaction does not yield formic acid. It would result in the formation of acetic acid.
In conclusion, the correct option to obtain formic acid from the given choices is option C, where glycerol is heated with oxalic acid at 373 K.

|
Explore Courses for Class 12 exam
|

|
Question Description
Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer?.
Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer?.
Solutions for Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Formic acid is obtained when [1994]a)Calcium acetate is heated with conc. H2SO4b)Calcium formate is heated with calciumacetatec)Glycerol is heated with oxalic acid at 373 Kd)Acetaldehyde is oxidised with K2Cr2O7 and H2SO4.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















