Class 12 Exam > Class 12 Questions > The OH group of an alcohol or the –COOH...
Start Learning for Free
The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]
- a)phosphorus pentachloride
- b)hypochlorous acid
- c)chlorine
- d)hydrochloric acid
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
The OH group of an alcohol or the –COOH groupof a carboxylic aci...
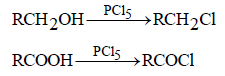
Among the given options, only PCl5 can
convert an alcoholic group as well as a
carboxyl group to chloride.
convert an alcoholic group as well as a
carboxyl group to chloride.
Most Upvoted Answer
The OH group of an alcohol or the –COOH groupof a carboxylic aci...
Phosphorus Pentachloride as a Reagent for Substitution Reactions
Phosphorus pentachloride (PCl5) is commonly used as a reagent for substitution reactions involving the replacement of hydroxyl (-OH) groups with chlorine. This reaction is especially common in the conversion of alcohols to alkyl chlorides.
Mechanism of the Reaction
- In the presence of PCl5, the lone pairs on the oxygen atom of the alcohol or carboxylic acid attack the electrophilic phosphorus atom in PCl5.
- This leads to the formation of an intermediate complex which then undergoes elimination of HCl to form the desired alkyl chloride or acyl chloride product.
Advantages of Using Phosphorus Pentachloride
- Phosphorus pentachloride is a cost-effective and readily available reagent.
- It allows for a rapid and efficient conversion of alcohols and carboxylic acids to their corresponding chlorides.
- The reaction conditions are relatively mild compared to other chlorinating agents.
Alternative Reagents
- Hypochlorous acid (HOCl) is not a suitable reagent for this type of substitution reaction as it is more commonly used as a bleaching agent.
- Chlorine gas and hydrochloric acid are not typically used for the direct substitution of hydroxyl groups with chlorine due to the harsh reaction conditions and potential side reactions that may occur.
In conclusion, phosphorus pentachloride is the most suitable reagent for replacing the -OH group of an alcohol or the -COOH group of a carboxylic acid with chlorine due to its efficiency, mild reaction conditions, and availability.
Phosphorus pentachloride (PCl5) is commonly used as a reagent for substitution reactions involving the replacement of hydroxyl (-OH) groups with chlorine. This reaction is especially common in the conversion of alcohols to alkyl chlorides.
Mechanism of the Reaction
- In the presence of PCl5, the lone pairs on the oxygen atom of the alcohol or carboxylic acid attack the electrophilic phosphorus atom in PCl5.
- This leads to the formation of an intermediate complex which then undergoes elimination of HCl to form the desired alkyl chloride or acyl chloride product.
Advantages of Using Phosphorus Pentachloride
- Phosphorus pentachloride is a cost-effective and readily available reagent.
- It allows for a rapid and efficient conversion of alcohols and carboxylic acids to their corresponding chlorides.
- The reaction conditions are relatively mild compared to other chlorinating agents.
Alternative Reagents
- Hypochlorous acid (HOCl) is not a suitable reagent for this type of substitution reaction as it is more commonly used as a bleaching agent.
- Chlorine gas and hydrochloric acid are not typically used for the direct substitution of hydroxyl groups with chlorine due to the harsh reaction conditions and potential side reactions that may occur.
In conclusion, phosphorus pentachloride is the most suitable reagent for replacing the -OH group of an alcohol or the -COOH group of a carboxylic acid with chlorine due to its efficiency, mild reaction conditions, and availability.

|
Explore Courses for Class 12 exam
|

|
Question Description
The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer?.
The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer?.
Solutions for The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The OH group of an alcohol or the –COOH groupof a carboxylic acid can be replaced by–Cl using [2004]a)phosphorus pentachlorideb)hypochlorous acidc)chlorined)hydrochloric acidCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















