Class 12 Exam > Class 12 Questions > In a photo-emissive cell, with exciting wavel...
Start Learning for Free
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed to 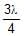 the kinetic energy of the fastest emitted electron will be :
the kinetic energy of the fastest emitted electron will be :
- a)3K/4
- b)4K/3
- c)Less than 4K/3
- d)Greater than 4K/3
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
In a photo-emissive cell, with exciting wavelength l, the maximum kine...
From E=W0+(1/2)mvmax2⇒vmax= √[(2E/m)−(2W0/m)]
where E= hc/λ
If wavelength of incident light charges from λ to 3λ/4 (Decreases)
Let energy of incident light charges from E and speed of fastest electron changes from v to v′ then
v′=√[(2E′/m)−(2W0/m)]
As E∝1/λ⇒E′(4/3)E
Hence v′=√[(2(4/3)E/m)−(2W0/m)]
⇒v′=(4/3)1/2 [(2E/m)− (2W0/m(4/3)1/2)]
So, v′>(4/3)1/2v
where E= hc/λ
If wavelength of incident light charges from λ to 3λ/4 (Decreases)
Let energy of incident light charges from E and speed of fastest electron changes from v to v′ then
v′=√[(2E′/m)−(2W0/m)]
As E∝1/λ⇒E′(4/3)E
Hence v′=√[(2(4/3)E/m)−(2W0/m)]
⇒v′=(4/3)1/2 [(2E/m)− (2W0/m(4/3)1/2)]
So, v′>(4/3)1/2v
Most Upvoted Answer
In a photo-emissive cell, with exciting wavelength l, the maximum kine...
From E=W0+(1/2)mvmax2⇒vmax= √[(2E/m)−(2W0/m)]
where E= hc/λ
If wavelength of incident light charges from λ to 3λ/4 (Decreases)
Let energy of incident light charges from E and speed of fastest electron changes from v to v′ then
v′=√[(2E′/m)−(2W0/m)]
As E∝1/λ⇒E′(4/3)E
Hence v′=√[(2(4/3)E/m)−(2W0/m)]
⇒v′=(4/3)1/2 [(2E/m)− (2W0/m(4/3)1/2)]
So, v′>(4/3)1/2v
where E= hc/λ
If wavelength of incident light charges from λ to 3λ/4 (Decreases)
Let energy of incident light charges from E and speed of fastest electron changes from v to v′ then
v′=√[(2E′/m)−(2W0/m)]
As E∝1/λ⇒E′(4/3)E
Hence v′=√[(2(4/3)E/m)−(2W0/m)]
⇒v′=(4/3)1/2 [(2E/m)− (2W0/m(4/3)1/2)]
So, v′>(4/3)1/2v
Free Test
FREE
| Start Free Test |
Community Answer
In a photo-emissive cell, with exciting wavelength l, the maximum kine...
Here we need to apply some maths and equation of photoelectric effect.
As we know
(as my phone doesn't support some Greek letters, I
have used following letters instead of them
y wavelength
¢ work function)
hc/y = ¢ + k
(k denotes maximum kinetic energy correspondence to y
and K to that of 3y/4)
So initially the equation was like this
hc/y = ¢ + k ... 1
And after the change of wavelength
4hc/3y = ¢ + K ... 2
Substituting hc/y from eq 2 using equation 1,
4(¢+k) /3=¢+K
4¢/3 + 4k/3 - ¢ = K
¢/3 + 4k/3 = K
As ¢ is positive for every metal,
K would always be greater than 4k/3
As we know
(as my phone doesn't support some Greek letters, I
have used following letters instead of them
y wavelength
¢ work function)
hc/y = ¢ + k
(k denotes maximum kinetic energy correspondence to y
and K to that of 3y/4)
So initially the equation was like this
hc/y = ¢ + k ... 1
And after the change of wavelength
4hc/3y = ¢ + K ... 2
Substituting hc/y from eq 2 using equation 1,
4(¢+k) /3=¢+K
4¢/3 + 4k/3 - ¢ = K
¢/3 + 4k/3 = K
As ¢ is positive for every metal,
K would always be greater than 4k/3

|
Explore Courses for Class 12 exam
|

|
Question Description
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer?.
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer?.
Solutions for In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer?, a detailed solution for In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed tothe kinetic energy of the fastest emitted electron will be :a)3K/4b)4K/3c)Less than 4K/3d)Greater than 4K/3Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















