Class 12 Exam > Class 12 Questions > The vapour pressure of two liquids ‘P&r...
Start Learning for Free
The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]
- a)72 torr
- b)140 torr
- c)68 torr
- d)20 torr
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
The vapour pressure of two liquids ‘P’ an d ‘Q&rsquo...
Given V.PP = 80 torr V.PQ = 60 torr
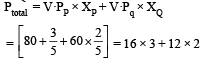
Ptotal = 48 + 24 = 72 torr
Most Upvoted Answer
The vapour pressure of two liquids ‘P’ an d ‘Q&rsquo...
The vapor pressure of a liquid is the pressure exerted by its vapor when it is in equilibrium with its liquid phase at a given temperature. It is a measure of how volatile a liquid is and is affected by factors such as temperature and intermolecular forces.
The vapor pressure of a liquid can vary depending on its chemical composition, temperature, and surrounding environment. Generally, liquids with weaker intermolecular forces and lower boiling points tend to have higher vapor pressures.
For example, at a given temperature, a liquid with a lower boiling point will have a higher vapor pressure compared to a liquid with a higher boiling point. This is because the weaker intermolecular forces in the low boiling point liquid allow more molecules to escape from the liquid phase and form a vapor.
Additionally, increasing the temperature of a liquid will also increase its vapor pressure. This is because higher temperatures provide more energy to the liquid molecules, making it easier for them to overcome the intermolecular forces and escape into the vapor phase.
In summary, the vapor pressure of a liquid is influenced by factors such as temperature and intermolecular forces, and it can vary depending on the specific characteristics of the liquid.
The vapor pressure of a liquid can vary depending on its chemical composition, temperature, and surrounding environment. Generally, liquids with weaker intermolecular forces and lower boiling points tend to have higher vapor pressures.
For example, at a given temperature, a liquid with a lower boiling point will have a higher vapor pressure compared to a liquid with a higher boiling point. This is because the weaker intermolecular forces in the low boiling point liquid allow more molecules to escape from the liquid phase and form a vapor.
Additionally, increasing the temperature of a liquid will also increase its vapor pressure. This is because higher temperatures provide more energy to the liquid molecules, making it easier for them to overcome the intermolecular forces and escape into the vapor phase.
In summary, the vapor pressure of a liquid is influenced by factors such as temperature and intermolecular forces, and it can vary depending on the specific characteristics of the liquid.

|
Explore Courses for Class 12 exam
|

|
Question Description
The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer?.
The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer?.
Solutions for The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]a)72 torrb)140 torrc)68 torrd)20 torrCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















