Acids, Bases and Salts Class 7 Notes Science Chapter 2
| Table of contents |

|
| Acids and Bases |

|
| Natural Indicators Around Us |

|
| Characteristics of Acids |

|
| Characteristics of Bases |

|
| Neutralisation |

|
| Neutralization in Everyday Life |

|
Substances can be divided into three types – Acid, Base, and Salt.
Acids and Bases
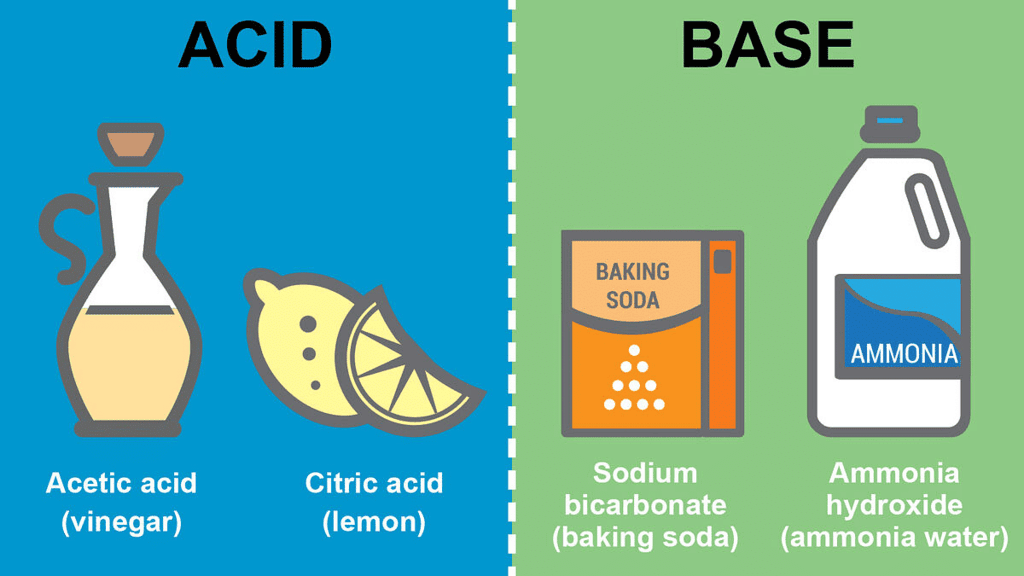
Acids
The taste of acid is sour. There are many substances that contain acid and so taste sour. For example – lemon, curd, pickles, orange juice, vinegar, etc.
Substances that taste sour and are corrosive in nature are called acids. The chemical nature of such a substance is known as acidic.
The word acid comes from the Latin ‘ACERE’, which means sour.
Bases
The taste of base is bitter. Bases are substances that, in an aqueous solution, are slippery to the touch and bitter in taste. For example; soap or soap solution, baking soda, washing soda, etc.
The chemical nature of substances that contain a base is known as basic.
Indicators
It is not always possible to know the acidic or basic nature of substances by tasting them. Tasting a substance in the laboratory is not also advisable because it may be harmful.
Thus, to test the chemical nature (acidic or basic nature) of a substance a special kind of substance is used. This special kind of substance which is used to test the acidic or basic nature of anything is known as an Indicator.
The indicator is a substance that shows the acidic or basic nature of a substance by change in its colour.
Types of Indicators
Indicators can be divided into two types.
Natural Indicator: Indicators that are obtained from naturally occurring substances are called natural indicators. Example: litmus, turmeric, china rose, etc.
Synthetic Indicator: Indicators that are made in a laboratory are called synthetic indicators. Example: phenolphthalein, methyl orange, etc.
 Examples of Indicators
Examples of Indicators
Natural Indicators Around Us
Litmus: A Natural Dye
Litmus is extracted from Lichens. Lichen is a composite organism. Lichens consist of fungi and algae living in a symbiotic relationship.
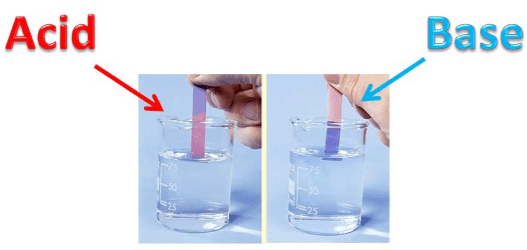
Litmus is a purple coloured liquid in distilled water. Litmus comes in the form of strips of two colours. One is called blue litmus paper and another is called red litmus paper.
Litmus liquid and litmus paper are used to detect the acidic or basic nature of a substance.
Colour of litmus paper in acid: Blue litmus paper turns red when dipped in acidic solution.
Colour of litmus paper in base: Red litmus paper turns blue when dipped in basic solution.
Turmeric is Another Natural Indicator
Turmeric is used as another natural indicator. Turmeric is of yellow colour. Turmeric paper turns red when it is dipped into a basic solution. Turmeric paper does not change its colour with acid.
China Rose as Indicator
China rose is another natural indicator. China rose solution gives dark pink (magenta) colour with acid and green colour with base.
Acid Rain
Carbon dioxide, sulphur dioxide and nitrogen dioxide which are released from vehicles and chimneys mix with droplets of rain and turn the rain water acidic. When this acidic rain water falls over earth, it is known as acid rain. Acid rain damages the buildings and is harmful for plants and animals.
Taj Mahal, which is made of marble is in threat because of acid rain. Many parts of Taj Mahal and other many historical buildings and monuments have got damaged due to acid rain.
Characteristics of Acids
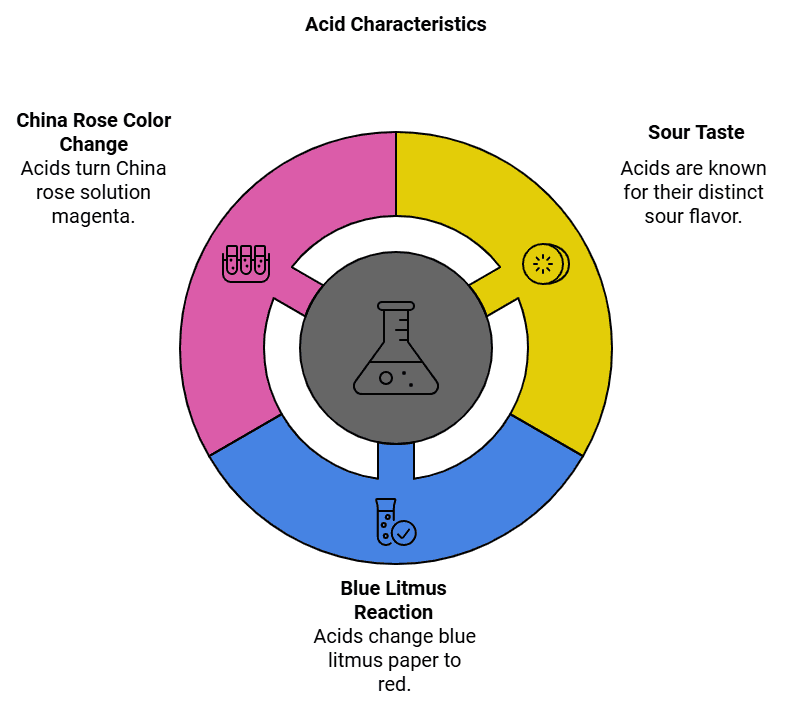
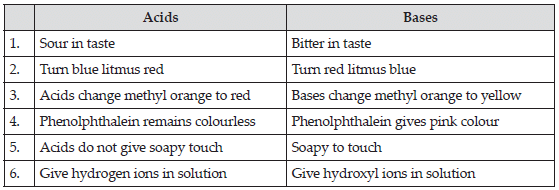
- Sour in taste.
- Turns blue litmus paper red.
- Turns the solution of China rose to dark pink colour (magenta).
Characteristics of Bases
- Bitter in taste.
- Turns red litmus paper blue.
- Turns solution of China rose to green.
- Turns turmeric paper to red.
Neutralisation
When a solution of acid is mixed with the solution of a base, both of them neutralize each other and a third substance; called salt; is formed. Such a phenomenon is called neutralization or neutralization reaction. The solution formed because of the mixing of the solution of acid and base is neither acidic nor basic in nature. Such a solution is known as a neutral solution.
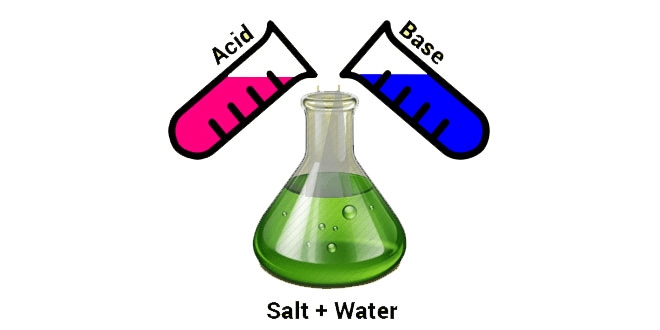 Neutralization
Neutralization
Salt formed because of neutralization reaction may be acidic or basic in nature. Acidic or Basic nature of salt depends upon the strength of acid and base. In neutralization reaction heat is evolved. Reactions in which heat is evolved are known as exothermic reactions. Thus, neutralization reaction is an exothermic reaction.
Sodium hydroxide is a base and hydrochloric acid is an acid. When solution of sodium hydroxide is mixed with the solution of hydrochloric acid, both neutralize each other and common salt (Sodium chloride) is formed. Since it is an exothermic reaction, so reaction mixture becomes slightly hot. The reaction involved in this can be written as follows:
Sodium chloride is the chemical name of common salt which is used in the household.
Neutralization in Everyday Life
There are many uses of neutralization reaction in everyday life:
Indigestion
Our stomach releases hydrochloric acid to kill bacteria; if any; present in food. Hydrochloric acid released in our stomach also helps in the digestion of food. Sometimes our stomach produces more hydrochloric acid than required. Production of more hydrochloric acid in the stomach manifests as indigestion. This condition can be painful and causes pain in the stomach.
To get rid of such symptoms, a medicine made of milk of magnesia (a base) is taken orally. Milk of magnesia, being a base neutralizes the hydrochloric acid and gives relief from pain because of indigestion.
Ant Bite
 Ant Bite
Ant Bite
Ant bite or bee sting contains methanoic acid. Methanoic acid is also known as formic acid. Bee or ant injects formic acid into our skin while biting. Injection of acid by ant or bee results in pain at the place of bite.
Rubbing baking soda over the skin gives relief from pain due to ant or bee sting. Baking soda, which is a base, neutralizes the effect of acid injected by bee or ant. Another base, such as zinc carbonate (Calamite solution) is also used in the case of ant or bee sting.
Soil Treatment
Sometimes soil becomes acidic or basic due to excess use of fertilizers or a wrong method of harvesting. Acidic or basic nature of soil affects the yield as plants do not grow properly on such soil. 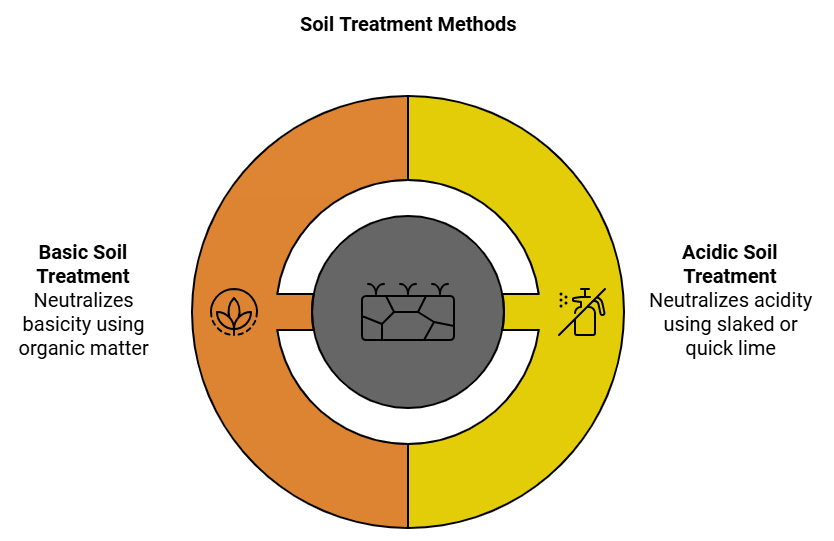
Acidic soil is treated with slaked lime or quick lime. Slaked lime (Calcium hydroxide) and quick lime (Calcium oxide) are bases. Use of slaked lime or quick lime neutralizes the acidic nature of the soil.
Basic soil is treated with organic matter. Organic matter releases acid and neutralizes the basic nature of the soil.
Factory Waste
Wastes of most of the factories are acidic. If such acidic wastes are flushed into rivers, the acid present in them kills the aquatic organisms and pollutes the water. Thus, factory wastes are treated with basic substances to neutralize the acid present in them before being flushed into the river.
|
111 videos|435 docs|28 tests
|
FAQs on Acids, Bases and Salts Class 7 Notes Science Chapter 2
| 1. What are the main characteristics of acids? |  |
| 2. What are the characteristics of bases? |  |
| 3. How does neutralization work? |  |
| 4. Can you provide examples of neutralization in everyday life? |  |
| 5. What natural indicators can be used to test for acids and bases? |  |





















