Cell Physiology and Membrane Potential Chapter Notes | Physiology - NEET PG PDF Download
Introduction to Cells
Definition: Cells are the basic structural and functional units of living organisms, discovered by Robert Hooke in 1665.
Components:
- Cell Membrane: Outer boundary.
- Cytoplasm: Jelly-like material inside the cell.
- Nucleus: Control centre containing genetic material.
Cell Membrane
Structure:
Thickness: 7.5–10 nanometers.
Composition:
Lipids (40%):
- Phospholipids (25%): Predominantly phosphatidylcholine and sphingomyelin in the outer leaflet; phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol in the inner leaflet.
- Cholesterol (13%): Present in both leaflets, stabilizes membrane at 37°C, maintains permeability and fluidity.
- Other Lipids (4%): Triglycerides = 0%.
Proteins (55%):
- Integral Proteins: Embedded in the membrane via hydrophobic interactions, may span the membrane (e.g., ion channels, transport proteins, receptors, G proteins).
- Peripheral Proteins: Loosely attached via covalent bonds, electrostatic interactions, or hydrogen bonds with integral proteins.
- Carbohydrates (3%): Glycoproteins and glycolipids, externally located, contribute to membrane asymmetry.
Protein-to-Lipid Ratio: Approximately 1:1 in most membranes.
- Highest in inner mitochondrial membrane (3.2), sarcoplasmic reticulum (2.0), outer mitochondrial membrane (1.1).
- Lowest in myelin (0.23), mouse liver cells (0.85), human erythrocyte (1.1).
Membrane Asymmetry:
Due to unique protein orientations and external carbohydrate location.
Transport Across Membrane:
Lipid-Soluble Substances: O₂, CO₂, steroid hormones dissolve in the lipid bilayer and cross easily.
Water-Soluble Substances: Na⁺, Cl⁻, glucose, H₂O cross via water-filled channels, pores, or carriers.
Fluid Mosaic Model
- Proposed: By Singer and Nicolson in 1972.
- Description: Integral proteins are like icebergs in a fluid sea of phospholipids.
- Membrane Fluidity:
Factors Affecting:
Temperature: Higher temperatures increase fluidity; transition from ordered (gel-like) to disordered (fluid) state at the transition temperature (Tm).
Lipid Composition:
- Unsaturated fatty acyl chains increase fluidity.
- Longer, saturated fatty acids decrease fluidity, increasing Tm.
Cholesterol: Acts as a fluidity buffer.
- Below Tm: Increases fluidity.
- Above Tm: Decreases fluidity.
Membrane Repair:
- Micro-Injury: Rapid resealing via hydrophobic interactions (self-sealing).
- Macro-Injury: Calcium-dependent process.
Lateral Diffusion: Proteins diffuse laterally in the lipid matrix unless restricted, supporting the fluid mosaic model.
Cytoplasm
Composition: Jelly-like, 80% water, contains cytosol (clear liquid with dissolved proteins, electrolytes, glucose) and organelles.
Organelles and Functions
Nucleus:
- Features: Control center, contains DNA (genes).
- Functions: RNA synthesis, protein synthesis instruction, ribosome subunit formation, cell division control, hereditary information storage.
Ribosomes:
- Features: No limiting membrane, 65% RNA, 35% proteins.
- Functions: Protein synthesis.
Rough Endoplasmic Reticulum (RER):
- Features: Tubular with ribosomes on surface.
- Functions: Protein synthesis, degradation of damaged organelles.
Smooth Endoplasmic Reticulum (SER):
- Features: No ribosomes (agranular).
- Functions: Lipid and steroid synthesis, calcium storage/metabolism, detoxification of toxic substances.
Lysosomes:
- Features: Vesicular, form from Golgi apparatus, acidic interior (pH 5.0 vs. cytoplasm pH 7.2), contain >40 acid hydrolase enzymes.
- Functions: Intracellular digestion, degrade worn-out organelles, remove excess secretory products, bacteria; secrete perforin, granzymes, melanin, serotonin.
- Lysosomal Storage Diseases: Enzyme deficiencies (e.g., Fabry disease: α-galactosidase A deficiency; Gaucher disease: β-galactocerebrosidase deficiency).
Peroxisomes:
- Features: Similar to lysosomes, form by self-replication or budding from SER, contain oxidases (produce H₂O₂) and catalases (break down H₂O₂).
- Functions: Break down fatty acids, detoxify H₂O₂, utilize oxygen, accelerate gluconeogenesis, degrade purine to uric acid, form myelin and bile acids.
Mitochondria:
- Features: Sausage-shaped, outer membrane, inner membrane with cristae, number varies (<100 to several thousand, e.g., high in cardiomyocytes, low in adipocytes).
- Functions: ATP synthesis via oxidative phosphorylation, initiate/regulate apoptosis.
Centrosome:
- Features: Near nucleus, contains two centrioles and pericentriolar material, microtubule-organizing centers (MTOCs) with γ-tubulin.
- Functions: Chromosome movement during cell division, monitors cell division steps.
Cytoskeleton
Components:
Microtubules:
- Structure: Long, hollow, 5-nm walls, 15-nm cavity, made of α- and β-tubulin (13 subunits/ring), γ-tubulin aids formation.
- Features: Polar, assembly at '+' end, disassembly at '−' end, GTP-dependent formation.
- Functions: Intracellular transport, cilia/flagella, centriole-mitotic spindle.
- Inhibitors: Colchicine, vinblastine (prevent assembly); Paclitaxel (Taxol) (stabilizes, prevents organelle movement).
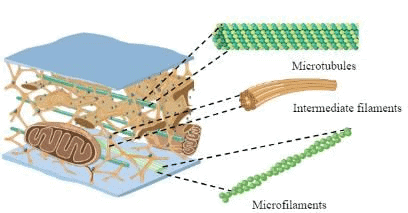
Microfilaments:
- Structure: Solid fibers of actin (15% of cell protein).
- Features: ATP-dependent polymerization/depolymerization, filamentous (F) actin vs. globular (G) actin.
- Functions: Cell junction, muscle contraction, slow axoplasmic transport, critical for contractile apparatus and locomotion.
Intermediate Filaments:
- Structure: Various subunits, connect nuclear to cell membrane.
- Features: Cell-type specific (e.g., vimentin: mesenchyme; cytokeratin: epithelial; glial fibrillary acidic protein: glial cells).
- Functions: Cell adhesion, cell shape.
- Note: Not directly involved in locomotion; absence causes cell rupture, abnormal filaments cause skin blistering.
Intercellular Junctions
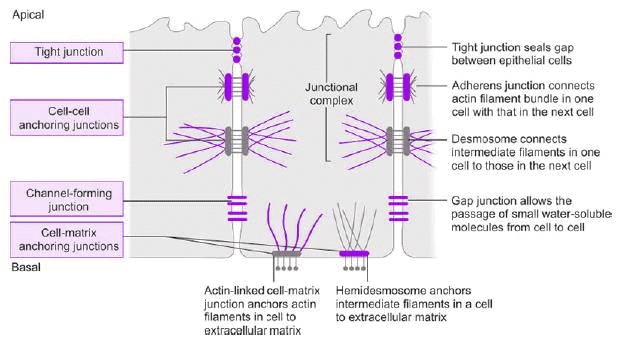 Types
Types
Tight Junction (Zonula Occludens):
- Location: Surrounds apical margins of epithelial cells.
- Proteins: Occludin, junctional adhesion molecules (JAMs), claudins.
- Function: Permits paracellular passage of some ions/solutes.
Adherens Junction (Zonula Adherens):
- Location: Basal to tight junction.
- Proteins: Cadherins (Ca²⁺-dependent).
- Function: Attaches microfilaments, signals during organ development/remodeling.
Desmosomes:
- Structure: Thickened membrane areas with intermediate filaments.
- Proteins: Desmoglein.
- Function: Structural support.
Hemidesmosomes:
- Structure: Half-desmosomes, attach to basal lamina.
- Proteins: Integrins.
- Function: Anchors cells to extracellular matrix.
Focal Adhesion:
- Structure: Labile, associated with actin filaments.
- Function: Cell movement, attachment to basal lamina.
Gap Junction:
- Structure: Narrows intercellular space (25 nm to 3 nm), formed by connexons (six connexin subunits).
- Function: Allows passage of ions, sugars, amino acids (MW <1000), electrically/metabolically couples cells.
- Regulation: By cytosolic Ca²⁺, cAMP, H⁺, membrane potential.
- Connexin Mutations: Cause diseases (e.g., Clouston syndrome, inherited deafness, cataracts, Charcot-Marie-Tooth disease).
Transport Across Cell Membrane
General Principles:
Molecules diffuse across lipid bilayers based on size and oil solubility.
Small Nonpolar Molecules: O₂, CO₂ diffuse rapidly.
Small Uncharged Polar Molecules: Water, urea diffuse slowly.
Charged Molecules/Ions: Highly impermeable due to charge and hydration.
Porins:
Beta barrel proteins in outer membranes of Gram-negative bacteria, mitochondria, chloroplasts.
Function: Passive diffusion of medium-sized/charged molecules (sugars, ions, amino acids).
Transport Proteins
Carrier Proteins (Transporters):
- Mechanism: Solute binds, transporter changes shape to move solute across.
Types:
- Facilitated Diffusion: No energy, down concentration gradient.
- Active Transport: Energy-dependent, against gradient.
Characteristics:
- Stereospecificity: e.g., D-glucose transported, not L-glucose.
- Saturation: Transport rate saturates at transport maximum (Tm).
- Competition: Related solutes compete (e.g., galactose inhibits glucose transport).
Channel Proteins:
Function: Allow rapid ion diffusion (Na⁺, K⁺, Cl⁻, Ca²⁺).
Features:
Selectivity: Based on channel diameter, charge, hydration (e.g., K⁺ channels selective for K⁺).
Gating: Open/closed states.
Ligand-Gated: Activated by molecule binding.
Voltage-Gated: Activated by membrane potential changes.
Mechanically Gated: Activated by membrane stretching.Structure: K⁺ channels and aquaporins are tetramers; ligand-gated channels have five subunits; Cl⁻ channels are dimers.
Transport Classification
Passive Transport:
(i) Simple Diffusion:
- No carrier, no Tm, follows Fick’s Law: ( J = -\frac{DA}{X} \Delta C ).
- Example: O₂/CO₂ exchange in alveoli.
(ii) Facilitated Diffusion:
- Carrier-mediated, no energy, saturable (has Tm), follows Michaelis-Menten kinetics.
- Example: Glucose transport via GLUT.
(iii) Non-Ionic Diffusion:
- Weak acids/bases cross in non-ionized form.
- Example: Ammonia transport in GIT/kidney.
Active Transport:
(i) Primary Active Transport:
Energy from transporter (e.g., Na⁺-K⁺ ATPase).
(ii) Secondary Active Transport:
Energy indirect (e.g., sodium-linked glucose transport, SGLT).
Transport Characteristics:
- Simple Diffusion: Downhill, no carrier, no energy, unaffected by Na⁺-K⁺ ATPase inhibition.
- Facilitated Diffusion: Downhill, carrier-mediated, no energy, unaffected by Na⁺-K⁺ ATPase inhibition.
- Primary Active Transport: Uphill, carrier-mediated, energy-dependent, inhibited by Na⁺-K⁺ ATPase inhibitors.
- Secondary Active Transport: Uphill (some solutes), carrier-mediated, energy-dependent, inhibited by Na⁺-K⁺ ATPase inhibitors.
Na⁺-K⁺ ATPase Pump
Structure: Heterodimer (α and β subunits).
α Subunits: α1, α2, α3; intracellular side binds ATP, 3 Na⁺; ECF side binds ouabain, 2 K⁺.
β Subunits: β1, β2, β3; 3 glycosylation sites on ECF side.
Function:
Electrogenic pump (3 Na⁺ out, 2 K⁺ in).
Regulates cell volume, pressure, maintains RMP (5–10%).
Regulation:
Stimulated by thyroid hormones, insulin, aldosterone, G-actin.
Inhibited by ouabain.
Osmosis
Definition: Water flow across a semipermeable membrane from low to high solute concentration.
Osmotic Pressure 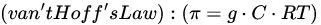
Modified:
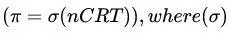 (reflection coefficient) accounts for membrane permeability.
(reflection coefficient) accounts for membrane permeability.(σ = 0): Freely permeable (e.g., urea, ineffective osmole).
(σ = 1): Impermeable (e.g., sucrose, effective osmole).
Example Calculation:
- RBC in 280 mOsm/kg H₂O (100 fl) moved to 350 mOsm/kg H₂O.
- Formula: (
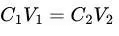 ).
). - Calculation:
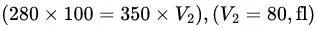 .
.
Oncotic Pressure:
- Osmotic pressure from large molecules (e.g., proteins).
- Normal plasma value: 26–28 mm Hg.
- Does not strictly follow van’t Hoff’s law for large proteins.
Exocytosis
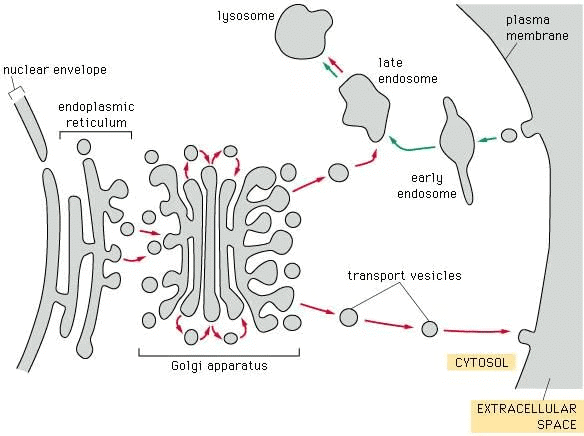 Definition: Delivery of secretory vesicle contents to extracellular fluid.
Definition: Delivery of secretory vesicle contents to extracellular fluid.
Mechanism: Vesicle membrane fuses with cell membrane, contents released, Ca²⁺-dependent.
Pathways:
- Nonconstitutive (Regulated): Proteins processed in secretory granules before release.
- Constitutive: Rapid transport to membrane with minimal processing.
Endocytosis
Definition: Uptake of extracellular material into the cell.
Types:
- Phagocytosis: Engulfs large particles (e.g., bacteria).
- Pinocytosis: Uptakes solutions in small vesicles.
Clathrin-Mediated (Receptor-Mediated):
- Occurs at clathrin-coated membrane indentations.
- Clathrin forms triskelion-shaped arrays, surrounds vesicle, then recycles.
- Internalizes receptors/ligands (e.g., nerve growth factor, low-density lipoproteins), important in synaptic function.
Resting Membrane Potential (RMP)
Definition: RMP is the voltage difference across a cell membrane at rest, where the extracellular surface has an excess of positive charge, and the cytoplasmic surface has an excess of negative charge.
Typical Value: In neurons, RMP is approximately -70 mV, close to the equilibrium potential of K⁺ ions.
Key Equations
Donnan Effect/Gibbs-Donnan Equilibrium
Nernst Equation
Goldman-Hodgkin-Katz (G-H-K) Equation (also known as Goldman constant field equation or chord conductance equation).
Donnan Effect/Gibbs-Donnan Equilibrium
Concept: Occurs due to the presence of a charged, impermeant ion (e.g., negatively charged proteins) on one side of a semipermeable membrane.
Repulsion and Attraction:
Repels similarly charged permeant ions (e.g., Cl⁻) to the opposite side.
Attracts oppositely charged permeant ions (e.g., Na⁺) to the same side.
Result: Asymmetric distribution of permeant ions across the membrane, leading to electroneutrality on each side.
Illustration:
Setup: A semipermeable membrane separates two solutions (A and B) of NaCl. Solution A contains non-diffusible negatively charged proteins.
Initial State: Equal Na⁺ concentrations in both solutions.
Equilibrium State:
Solution A: Higher Na⁺ (held by proteins), lower Cl⁻.
Solution B: Lower Na⁺, higher Cl⁻.
Each solution remains electroneutral (total cations = total anions).
Mathematical Expression (Gibbs-Donnan Equation):
Product of diffusible ion concentrations is equal on both sides:
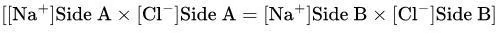
Each side is electrically neutral:
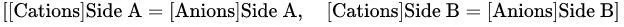
Osmotic Effect:
Side with impermeant ions (Side A) has higher osmolarity (e.g., 24 vs. 12 for Side B).
Relative Permeability of Molecules
Order of Permeability:
Hydrophobic Molecules (e.g., CO₂, O₂, N₂, steroid hormones) ≫ Small Uncharged Polar Molecules (e.g., H₂O, urea, glycerol) ≫ Large Uncharged Polar Molecules (e.g., glucose, sucrose) ≫ Ions (e.g., Na⁺, K⁺, Cl⁻).
Ion Permeability:
Synthetic Biological Membrane: Cl⁻ ≫ K⁺ ≫ Na⁺.
Cell Membrane: K⁺ ≫ Cl⁻ ≫ Na⁺.
Resting Condition Permeability Ratios: K⁺:Cl⁻:Na⁺ = 1.0:0.45:0.04.
Concept of Equilibrium Potential and RMP
Equilibrium Potential: The voltage across a membrane where the chemical and electrical driving forces for an ion are balanced, resulting in no net ion movement.
RMP Generation in Glial Cells:
Glial cells are permeable only to K⁺ at rest.
Mechanism:
High intracellular K⁺ concentration drives K⁺ efflux (chemical gradient).
Efflux leaves behind negative charges (impermeant anions like proteins), creating a positive extracellular surface.
At equilibrium, the electrical force (inward) balances the chemical force (outward), establishing the K⁺ equilibrium potential (E_K).
Result: RMP of glial cells equals EK (approximately -90 mV).
General Observation:
Most excitable cells are maximally permeable to K⁺ at rest, so RMP is close to EK.
In neurons, RMP (-70 mV) equals the equilibrium potential of Cl⁻.
Nernst Equation
Purpose: Calculates the equilibrium potential for a specific ion (Ex).
Formula:
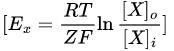
R: Gas constant.
T: Temperature (in Kelvin).
Z: Valence of the ion (e.g., +1 for Na⁺, -1 for Cl⁻, +2 for Ca²⁺).
F: Faraday constant.
$[X]_o$, $[X]_i$: Ion concentrations outside and inside the cell.
Simplified Form (at 37°C):
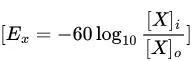
Conversion factor:
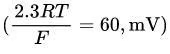 at 37°C.
at 37°C.
Example Calculation:
Given: Intracellular [Na⁺] = 15 mM, Extracellular [Na⁺] = 150 mM.
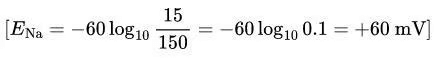
Intuitive Approach: Higher extracellular Na⁺ drives Na⁺ influx, making the inside positive (+60 mV).
Equilibrium Potentials for Ions:
Na⁺: +63 to +65 mV
K⁺: -90 to -96 mV
Cl⁻: -64 to -70 mV
Ca²⁺: +132 to +137 mV
H⁺: -12 mV
HCO₃⁻: -13 mV
Mg²⁺: +9 mV
RMP in Cells with Multiple Permeable Ions
Unlike Glial Cells: Most mammalian cells (e.g., neurons) are permeable to Na⁺, K⁺, and Cl⁻ at rest.
Net Ion Flow:
At RMP, the sum of ion currents is zero:
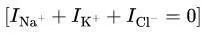
RMP settles where there is no net ion movement.
Dominance of K⁺:
Due to high K⁺ permeability, RMP is closest to EK (-90 mV).
Example: Neuron RMP (-70 mV) is influenced by K⁺, Cl⁻, and Na⁺, but dominated by K⁺.
Role of Na⁺-K⁺ ATPase Pump
Function: Maintains concentration gradients for Na⁺ and K⁺.
Pumps 3 Na⁺ out and 2 K⁺ in per ATP hydrolyzed.
Mechanism:
Creates high intracellular K⁺ and low intracellular Na⁺.
K⁺ efflux (via leak channels) is balanced by the electrical gradient pulling K⁺ inward.
Results in a slight excess of extracellular cations and intracellular anions, establishing RMP.
Significance: Maintains the conditions necessary for RMP stability.
Recording of RMP
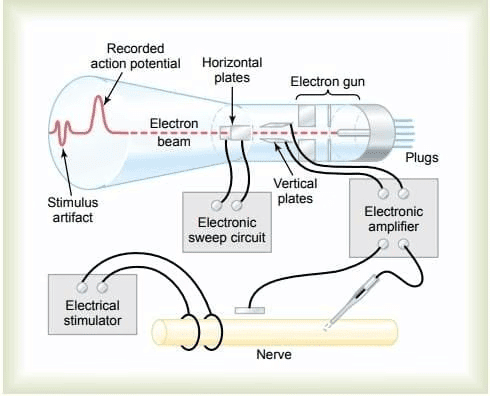 Method:
Method:
Two electrodes connected via an amplifier.
One electrode inside the cell, one outside.
Observation: Constant potential difference with the inside negative (e.g., -70 mV in neurons).
Electrode Details:
Tip diameter < 0.5 micrometers.
RMP and Threshold Voltage of Different Tissues
Calculation of RMP
Goldman-Hodgkin-Katz (G-H-K) Equation
Purpose: Calculates RMP based on ion concentrations and permeabilities.
Formula:
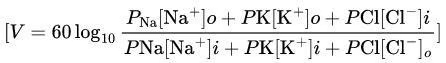
P: Permeability of the ion.
[]o, []i: Concentrations outside and inside the cell.
Dependencies:
Distribution of Na⁺, K⁺, and Cl⁻ between extracellular fluid (ECF) and intracellular fluid (ICF).
Relative permeabilities of the membrane to these ions.
Changes in RMP
Excitability: Ability of cells (neurons, muscle cells) to produce electrical signals via gated ion channels.
Types of Potentials
Graded Potentials:
Variable amplitude, conducted decrementally over short distances, no threshold/refractory period.
Types:
Receptor Potential: At afferent neuron endings due to stimuli.
Synaptic Potential: In postsynaptic neurons (EPSP: depolarizing, IPSP: hyperpolarizing).
Pacemaker Potential: Spontaneous in specialized cells (e.g., SA node).
Action Potentials: Brief, all-or-none, reverse polarity, have threshold/refractory period, conduct without decrement.
Terminology:
Depolarization: Membrane potential becomes less negative (e.g., -70 mV to -40 mV or +40 mV).
Repolarization: Returns to RMP after depolarization.
Hyperpolarization: Becomes more negative (e.g., -70 mV to -90 mV).
Overshoot: Inside becomes positive (>0 mV).
Effect of Ion Changes:
Hyperkalemia: Decreases RMP (e.g., -70 mV to -65 mV), due to reduced K⁺ efflux.
Hypokalemia: Increases RMP.
Hyponatremia/Hypernatremia: Minimal effect due to low Na⁺ permeability at rest.
Example Question:
If ECF K⁺ increases from 3.5 to 5 mM, adipose cell RMP becomes less negative (depolarizes) due to reduced K⁺ efflux, not K⁺ influx.
|
40 docs|9 tests
|
FAQs on Cell Physiology and Membrane Potential Chapter Notes - Physiology - NEET PG
| 1. What is the structure and function of the cell membrane? |  |
| 2. What is cytoplasm, and what role does it play in cellular function? |  |
| 3. How do intercellular junctions contribute to tissue integrity? |  |
| 4. What mechanisms are involved in transport across the cell membrane? |  |
| 5. What is resting membrane potential (RMP) and why is it important? |  |





















