Class 7 Exam > Class 7 Notes > Year 7 Chemistry (Cambridge) > Chapter Notes: Chemical Changes and Reactions
Chemical Changes and Reactions Chapter Notes | Year 7 Chemistry (Cambridge) - Class 7 PDF Download
Making compounds
Word Equations:
- In a chemical reaction, substances (reactants) react together to form new substances (products).
- A word equation is a model that represents a chemical reaction using the names of reactants and products.
- Example: magnesium + oxygen → magnesium oxide
- Reactants are written on the left, products on the right, and an arrow shows the direction of the reaction.
Particle Model:
- A particle diagram is another way to represent chemical reactions.
- It shows atoms as circles, and how they are arranged in reactants and products.
- The same number of each type of atom must be present on both sides of the diagram.
- Particle diagrams provide more information about the arrangement of atoms than word equations.
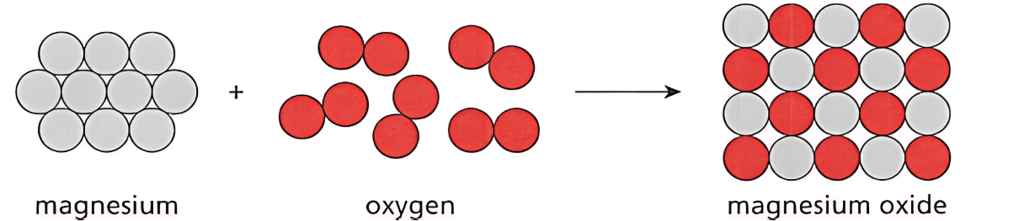 Particle Diagram
Particle Diagram
Making Compounds:
- Magnesium and oxygen are elements that react to form the compound magnesium oxide.
- Compounds contain atoms of different elements strongly held together.
- Properties of compounds differ from their constituent elements.
- Compounds can only be separated into elements through chemical reactions.
Identifying Chemical Reactions:
- Temperature changes, reactants being used up, products being formed, color changes, and precipitation can indicate a chemical reaction.
- Signs include disappearance of reactants, gas bubbles, color changes, and formation of a solid precipitate.
Testing for Hydrogen:
- Some reactions produce hydrogen gas.
- To test for hydrogen, hold a lighted splint near the gas – it will ignite with a 'pop' sound if hydrogen is present.
- Hydrogen is flammable and burns quickly.
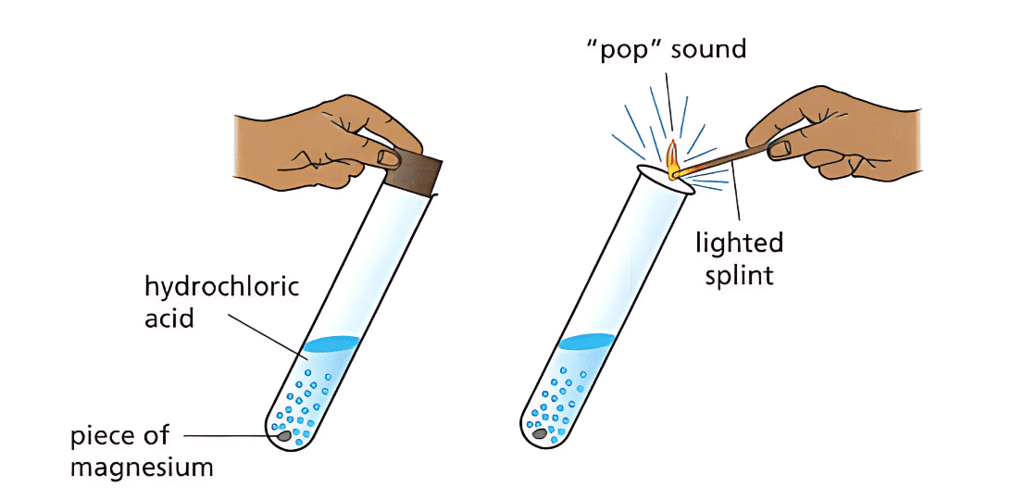 Testing for Hydrogen
Testing for Hydrogen
Testing for Oxygen:
- Reactions can produce oxygen gas.
- To test, hold a glowing splint just inside the gas – it will relight if oxygen is present.
- Oxygen allows the hot wood to start burning again.
Testing for Carbon Dioxide:
- Carbon dioxide gas can be produced in reactions.
- Pass the gas through limewater (calcium hydroxide solution) – it will turn milky white if carbon dioxide is present.
Limewater turns Milky
Question for Chapter Notes: Chemical Changes and ReactionsTry yourself: Which model provides more information about the arrangement of atoms in a chemical reaction?View Solution
Identifying Risks:
- Experiments may involve hazards (substances or procedures that can cause harm).
- Hazard labels indicate potential risks.
- Identify hazards and plan to control risks (reduce chances of harm) before starting an experiment.
Forming Precipitates
Soluble and Insoluble Substances:
- Some substances are soluble in water, meaning they can dissolve to form a clear solution.
- Example: Sodium chloride (table salt) is soluble in water and forms a clear solution.
- Solutions can be colorless (like sodium chloride solution) or colored (like blue copper sulfate solution).
- Other substances are insoluble in water, meaning they do not dissolve and cannot form a solution.
- If an insoluble substance is mixed with water, it remains visible, making the mixture cloudy.
- Example: Calcium carbonate is an insoluble white solid that makes water cloudy when mixed.
Precipitates:
- Substances dissolved in water can still participate in reactions (like acids).
- When soluble reactants react and form one or more insoluble products, an insoluble product formed is called a precipitate.
- A precipitate cannot dissolve in the reaction mixture, causing it to turn cloudy.
- A reaction in which a precipitate forms is called a precipitation reaction.
Modelling Precipitation Reactions:
- Word equations and particle diagrams can be used to model precipitation reactions.
- Example word equation: copper sulfate + sodium silicate → copper silicate + sodium sulfate
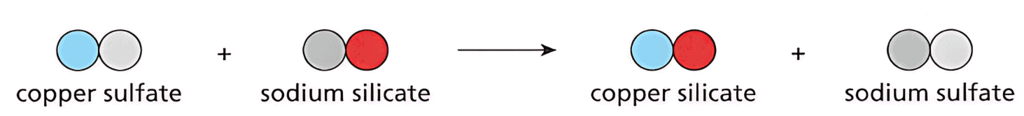
- Particle diagrams show the arrangement of particles but have limitations:
- They may not show all atoms present in the reaction mixture.
- They do not show the forces holding particles together or indicate solubility/insolubility.
- While these models provide some information, they have limitations in representing all aspects of precipitation reactions.
Question for Chapter Notes: Chemical Changes and ReactionsTry yourself: What is a hazard label used for in experiments?View Solution
Neutralisation Reactions
Measuring and Estimating pH:
- pH of a solution can be measured using a pH meter, which consists of a pH probe connected to a meter.
- Indicators are substances that change color depending on the pH of a solution.
- Universal Indicator is a mixture of indicators that changes color across the pH scale, from red to purple.
- Universal Indicator is available as a solution or test paper.
- To estimate pH using Universal Indicator, add a few drops of the solution or a small drop on test paper.
- Match the resulting color to a pH color chart to estimate the pH value.
What is Neutralization?
- When an acid is added to an alkali (base), a chemical reaction called neutralization occurs.
- In a neutralization reaction, water and another substance (which may dissolve in water) are formed.
- During neutralization, the pH of the solution changes.
- As an alkali is added to an acidic solution, the pH increases toward 7 (neutral).
- When the pH reaches 7, a neutral solution is formed.
- If more alkali is added after reaching pH 7, the solution becomes alkaline, and the pH increases above 7.
- Neutralization involves the gradual change of an acidic solution to a basic solution (or vice versa) as the neutralizing agent is added.
The document Chemical Changes and Reactions Chapter Notes | Year 7 Chemistry (Cambridge) - Class 7 is a part of the Class 7 Course Year 7 Chemistry (Cambridge).
All you need of Class 7 at this link: Class 7
|
7 videos|15 docs|8 tests
|
FAQs on Chemical Changes and Reactions Chapter Notes - Year 7 Chemistry (Cambridge) - Class 7
| 1. What are some examples of compounds that can be formed through chemical reactions? |  |
Ans. Compounds like water (H2O), salt (NaCl), and carbon dioxide (CO2) are examples of compounds that can be formed through chemical reactions.
| 2. How do precipitates form during chemical reactions? |  |
Ans. Precipitates form when two solutions are mixed together and a solid substance is produced as a result of a chemical reaction. This solid substance is insoluble in the solution and settles at the bottom of the container.
| 3. What happens during a neutralization reaction? |  |
Ans. In a neutralization reaction, an acid and a base react to form a salt and water. The pH level of the solution becomes neutral (pH 7) as a result of this reaction.
| 4. Can compounds be broken down into their original elements through chemical reactions? |  |
Ans. Yes, compounds can be broken down into their original elements through chemical reactions. This process is known as decomposition and involves breaking the bonds between the elements in the compound.
| 5. How can you tell if a chemical reaction has occurred? |  |
Ans. Some signs that a chemical reaction has occurred include the formation of a precipitate, a change in color, the release of gas, or a change in temperature. These are all indications that a chemical change has taken place.
Related Searches
















