Gaseous Exchange Chapter Notes | Physiology - NEET PG PDF Download
Physical Principles of Gas Exchange
- Human lungs contain approximately 300 alveoli, each with an average diameter of 0.2 mm.
- Alveolar walls are extremely thin, allowing close proximity between alveolar gases and pulmonary capillary blood.
- Gas exchange occurs across the respiratory membrane, which includes the respiratory bronchiole, alveolar ducts, atria, and alveoli, not just the alveoli.
- The respiratory membrane, also known as the pulmonary membrane, consists of:
- A thin layer of fluid lining the alveolus.
- Alveolar epithelium cells.
- Basement membrane of epithelial cells.
- A thin interstitial space between the alveolar epithelium and capillary membrane.
- Capillary basement membrane.
- Endothelial cells of the capillary.
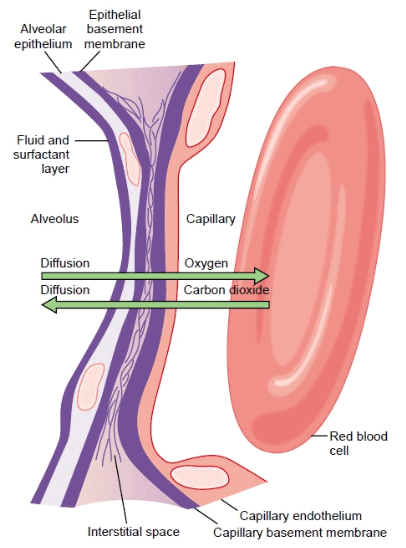 Ultrastructure of the alveolar respiratory membrane and direction of gas exchange.
Ultrastructure of the alveolar respiratory membrane and direction of gas exchange.
- The average thickness of the respiratory membrane is 0.5 μm (range: 0.2 to 0.6 μm).
- The total surface area of the respiratory membrane in a healthy adult male is approximately 70 m².
- Respiratory membrane thickness may increase due to interstitial edema or lung fibrosis.
- The surface area of the respiratory membrane can decrease due to conditions like pneumonectomy or emphysema (caused by the rupture of many alveoli).
- The rate of diffusion (D) is governed by the formula:
- D ∝ (ΔP × A × S) / (d × √M), where:
- ΔP: Partial pressure difference between the two sides of the diffusion pathway.
- A: Cross-sectional area of the pathway.
- S: Solubility of the gas.
- d: Distance of diffusion.
- M: Molecular weight of the gas.
- D ∝ (ΔP × A × S) / (d × √M), where:
- The diffusion coefficient of a gas is proportional to S / √M, determined by its solubility (S) and molecular weight (M).
- Assuming the diffusion coefficient of oxygen is 1, the relative diffusion coefficient for CO₂ is ~20, and for carbon monoxide (CO) is 0.81.
- CO₂ diffuses approximately 20 times faster through the respiratory membrane than O₂ at the same partial pressure due to its higher diffusion coefficient.
- Lung disorders often result in hypoxemia (low blood O₂ content) rather than hypercarbia (CO₂ retention) because CO₂ diffuses more readily.
- Alveolar diffusion decreases in emphysema due to reduced effective surface area (A) of the respiratory membrane.
- Diffusion is also reduced in diseases that increase the diffusion distance (d), such as edema in acute lung injury or fibrosis in interstitial lung diseases.
Capillary Transit Time
- A red blood cell takes approximately 4 to 5 seconds to travel through the pulmonary circulation at resting cardiac output, with ~0.75 seconds (≈0.8 seconds) spent in the alveolar capillary.
- The time a red blood cell spends in the pulmonary capillaries is called the capillary transit time.
- Capillary transit time is calculated using the formula:
- Mean capillary transit time = (Pulmonary capillary blood volume) / (Pulmonary blood flow).
- Total blood volume in pulmonary circulation is approximately 450 mL, with 70 mL (range: 60 to 140 mL) located in the pulmonary capillaries.
- Pulmonary blood flow (PBF) is nearly equal to cardiac output (CO), approximately 5.4 L/min (90 mL/sec) at rest.
- Normal capillary transit time at rest is approximately 0.8 seconds, calculated from the above formula.
- An increase in cardiac output reduces capillary transit time.
- Transit time is critical for determining pulmonary end-capillary PO₂ and diffusing capacity, as it allows partial pressure equilibration between blood and alveolar gases.
Perfusion-Limited and Diffusion-Limited Gas
- Figure the uptake of N₂O, O₂, and CO in blood relative to their partial pressures and red blood cell transit time in the capillary.
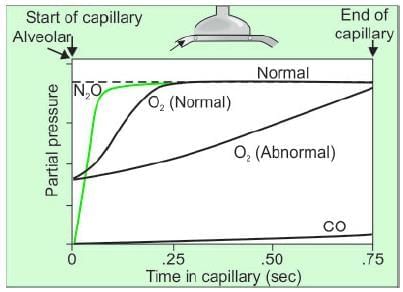
- Perfusion-limited gases (e.g., N₂O and O₂) reach equilibrium with alveolar pressure before exiting the capillary.
- Diffusion-limited gases (e.g., CO) do not reach equilibrium with alveolar pressure during transit time.
- Normally, oxygen diffusion equilibrium occurs within 0.25 seconds (1/3 of total transit time), providing a three-fold safety factor.
- Even if transit time decreases to 0.25 seconds due to high cardiac output, oxygen diffusion equilibrium is achieved.
- In lung diseases like fibrosis, reduced diffusion capacity may prevent capillary PO₂ from equilibrating with alveolar PO₂ during transit time.
- N₂O is highly soluble in blood but does not bind to hemoglobin, leading to rapid partial pressure rise and quick diffusion equilibrium regardless of transit time.
- Changes in diffusing capacity do not affect N₂O uptake; increased uptake requires increased perfusion or cardiac output.
- N₂O uptake is limited by perfusion, as more red blood cells passing through alveolar capillaries increase uptake.
- Carbon monoxide (CO) has a 250 times greater affinity for hemoglobin than oxygen, preventing significant partial pressure rise in capillary blood.
- CO diffusion equilibrium is not achieved during transit time, and uptake depends on diffusing capacity rather than perfusion.
- Increasing cardiac output does not significantly increase CO uptake.
- All gas transport in the lung (e.g., O₂, CO₂) is perfusion-limited, except for CO and O₂ under hypoxic conditions.
- Diffusion-limited gases include CO and O₂ in hypoxic conditions; all other gases, including O₂ under normoxic conditions in healthy lungs, are perfusion-limited.
Diffusing Capacity of Respiratory Membrane
- Diffusing capacity is defined as the volume of gas that diffuses through the respiratory membrane per minute for a partial pressure difference of 1 mm Hg.
- Diffusing capacity is directly proportional to the surface area of the alveolocapillary membrane and inversely proportional to its thickness.
- Diffusing capacity for carbon monoxide (DLCO) is measured as an index of diffusing capacity since CO uptake is diffusion-limited.
- The general equation for DLCOis:
- DLCO = (CO uptake in the blood) / (Partial pressure of CO in the alveoli - Partial pressure of CO in blood).
- CO binds to hemoglobin with high affinity, making the partial pressure of CO in blood effectively zero, except in habitual smokers.
- The simplified DLCOequation is:
- DLCO = (CO uptake in the blood) / (Partial pressure of CO in the alveoli).
- The single-breath method, using a gas mixture with 0.3% CO and 10% helium, is the most common protocol for measuring DLCO.
- Normal DLCO at rest is approximately 17 mL/min/mm Hg.
- Oxygen diffusion capacity is 1.23 times that of CO (due to a diffusion coefficient 1.23 times higher), ranging from 21 to 25 mL/min/mm Hg.
- CO₂ diffusion capacity is approximately 400 to 450 mL/min/mm Hg at rest, increasing to 1200 mL/min/mm Hg during exercise, due to its diffusion coefficient being ~20 times that of O₂.
- Causes of high and low DLCO:
- Causes of High D_LCO:
- Recruitment of blood in alveolar capillary bed (e.g., supine position, hyperdynamic circulation like fever or exercise, bronchial asthma, Muller’s maneuver, left-to-right cardiac shunting, early congestive failure).
- Miscellaneous causes: polycythemia, alveolar hemorrhage, obesity (cause uncertain), high altitude (due to low FiO₂, increased CO binding with hemoglobin), post-bronchodilation in obstructive disease (6% increase).
- Causes of Low D_LCO:
- Decreased surface area for diffusion (e.g., pulmonary resection, emphysema, V/Q mismatch due to pulmonary obstruction).
- Alveolar capillary membrane disease (e.g., interstitial lung disease like sarcoidosis or connective tissue disease, pulmonary vascular disease like embolism, pulmonary hypertension, vasculitis, alveolar edema, cardiac insufficiency).
- Miscellaneous causes: anemia, lung cancer, chronic bronchitis, cystic fibrosis.
Gas Transport
Symbols used: P (partial pressure), I (inspired air), E (expired air), A (alveolar), a (arterial), v (venous), v̅ (mixed venous). For example, PIO₂ is the partial pressure of O₂ in inspired air.
Composition of Atmospheric and Alveolar Gases:
- Atmospheric air exerts barometric pressure (Pbaro) of 736 mm Hg at sea level.
- Dalton’s law states that Pbaro equals the sum of partial pressures of individual gases: Pbaro = PN2 + PO2 + PCO2.
- Partial pressure of O₂ (PO2) is calculated as: PO2 = Pbaro × FO2, where FO2 is the fractional concentration of oxygen (21%). Thus, PO2 = 160 mm Hg (736 × 0.21).
- Inspired air is warmed and humidified, adding water vapor with a partial pressure (PH2O) of 47 mm Hg at 37°C, independent of barometric pressure.
- Water vapor reduces the total pressure of dry gases in the conducting zone to (Pbaro - PH2O) = (736 - 47) = 689 mm Hg.
- The formula for partial pressure of inspired air (Pi) is: Pi = (Pbaro - PH2O) × Fi, where Fi is the fraction of inspired dry air (FIO2 = 21%, FIN2 = 79%).
- Partial pressures in inspired air: PIO2 = (736 - 47) × 0.21 = 145 mm Hg; PIN2 = (736 - 47) × 0.79 = 545 mm Hg.
- Multicompartment model shows gas compositions:
- Atmospheric air: N₂ (78.6%, 597 mm Hg), O₂ (20.8%, 159 mm Hg), CO₂ (0.04%, 0.3 mm Hg), H₂O (0.5%, 3.7 mm Hg).
- Humidified air: N₂ (563 mm Hg), O₂ (149 mm Hg), CO₂ (0.3 mm Hg), H₂O (47 mm Hg).
- Alveolar air: N₂ (569 mm Hg), O₂ (104 mm Hg), CO₂ (40 mm Hg), H₂O (47 mm Hg).
- Expired air: N₂ (566 mm Hg, 75%), O₂ (120 mm Hg, 16%), CO₂ (27 mm Hg, 4%), H₂O (47 mm Hg, 6%).
- Alveolar Gas Equation:
- Simplified alveolar gas equation for oxygen partial pressure in alveoli (PAO2): PAO2 = PIO2 - (PACO2/ R.Q) + F, where:
- PACO2: Partial pressure of CO₂ in alveoli (normally 40 mm Hg).
- R.Q: Respiratory quotient (CO₂ production / O₂ consumption), determined by tissue metabolism in a steady state.
- F: Small correction factor (~2 mm Hg for air breathing), often ignored.
- When R.Q = 1, PAO2 = PIO2 - PACO2 = 145 - 40 = 105 mm Hg.
- Simplified alveolar gas equation for oxygen partial pressure in alveoli (PAO2): PAO2 = PIO2 - (PACO2/ R.Q) + F, where:
Physiological Shunt
- Arterial blood partial pressure of O₂ (PaO₂) is 95-98 mm Hg, lower than alveolar PaO2 (100-104 mm Hg) due to venous admixture or physiological shunt.
- Physiological shunt occurs when well-oxygenated blood from alveoli mixes with less oxygenated blood, primarily from bronchial circulation.
- Bronchial arteries, branches of the aorta, supply conducting airways with oxygenated blood, and their capillaries anastomose with pulmonary artery-derived capillaries at the respiratory bronchioles, draining partially into pulmonary veins.
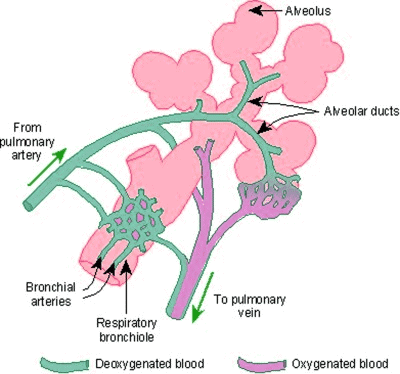 Physiological shunt.
Physiological shunt. - This mixing of partially deoxygenated bronchial blood with oxygenated blood causes a slight reduction in final PO2 compared to alveolar PO2.
- Shunt flow is calculated based on oxygen content: QT × CaO2 = QS × CvO2 + (QT - QS) × CcO2, where:
- QT: Total blood flow (cardiac output).
- CaO2: Oxygen concentration in arterial blood.
- QS: Shunted blood flow.
- CvO2: Oxygen concentration in venous blood.
- CcO2: Oxygen concentration in end-capillary blood.
- Rearranged shunt equation: QS / QT = (CcO2 - CaO2) / (CcO2 - CvO2).
- Normal physiological shunt is ~2% of total cardiac output (QT).
- Shunt fraction increases in pathological conditions like patent ductus arteriosus (PDA) or patent foramen ovale (PFO), allowing systemic venous blood to bypass the lungs.
- Shunt fractions approaching 50% are life-threatening, requiring aggressive management, such as mechanical ventilation with positive end-expiratory pressure (PEEP) to recruit consolidated or atelectatic alveoli.
- Hypoxemia in pathological shunts responds poorly to 100% O₂ breathing, as shunted blood bypasses ventilated alveoli, though some arterial PO2 elevation occurs due to oxygen added to ventilated lung capillary blood.
O₂ Transport
Oxygen transport from lungs to tissues occurs via blood circulation and diffusion along a concentration gradient, represented by PO2 differences:
- Atmospheric PO2: 158-160 mm Hg.
- Inspired air PO2: 149 mm Hg.
- Alveolar air PO2: 100 mm Hg.
- Arterial blood PO2: 95 mm Hg.
- Venous blood PO2: 40 mm Hg.
- Tissue interstitial fluid PO2: 40 mm Hg.
- The gradual decline in PO2 from atmosphere to tissues is called the oxygen cascade.
- Oxygen exchange from alveolar air to pulmonary capillary blood and from arterial blood to tissues occurs by simple diffusion.
- Oxygen transport involves three processes:
Uptake of oxygen by pulmonary blood:
- Alveolar air PO2 is 100 mm Hg, while pulmonary arterial blood PO2 is ~40 mm Hg, driving oxygen diffusion into the blood.
Transport of oxygen in arterial blood:
Oxygen is transported in two forms:
- Hemoglobin-bound O₂ (99%).
- Dissolved O₂ (1%).
- Hemoglobin-bound O₂:
- Depends on hemoglobin saturation (SO2 or SpO₂), which depends on P_O2.
- At PO2 = 120 mm Hg, hemoglobin is 100% saturated, carrying 1.39 mL O₂/g of pure hemoglobin (oxygen capacity).
- In reality, oxygen capacity is ~1.34 mL O₂/g hemoglobin due to inactive hemoglobin derivatives (e.g., methemoglobin, carboxyhemoglobin).
- 15 g hemoglobin carries 20.1 mL O₂ when fully saturated (1.34 × 15).
- In arterial blood (PaO₂ = 95 mm Hg), hemoglobin is 97% saturated, carrying 19.5 mL O₂/100 mL blood (20.1 × 97/100).
- In venous blood (PvO₂ = 40 mm Hg), hemoglobin is 75% saturated, carrying 15.1 mL O₂/100 mL blood.
- Dissolved O₂:
- Obeys Henry’s law: Amount dissolved is proportional to P_O2.
- Dissolved O₂ per 100 mL blood = Solubility of O₂ × P_O2, where solubility is 0.003 mL/mm Hg/dL.
- In arterial blood (PaO₂ = 95 mm Hg), dissolved O₂ = 0.003 × 95 = 0.285 mL/100 mL.
- In venous blood (PvO₂ = 40 mm Hg), dissolved O₂ = 0.003 × 40 = 0.12 mL/100 mL.
Total O₂ in 100 mL blood:
- Arterial blood: 19.5 (Hb-bound) + 0.285 (dissolved) = 19.8 mL.
- Venous blood: 15.1 (Hb-bound) + 0.12 (dissolved) = 15.2 mL.
Release of oxygen to tissues:
- Tissues consume ~5 mL O₂/100 mL arterial blood at rest, with a total body oxygen consumption of 250 mL/min (given a cardiac output of 5 L/min).
- Utilization coefficient = (Oxygen consumed/min) / (Oxygen delivered/min) × 100 = (250 mL/min) / (1000 mL/min) × 100 = 25%.
- Arterial blood carries ~19.8 mL O₂/100 mL, while venous blood carries ~15.2 mL, delivering ~4.6 mL O₂/100 mL to tissues (0.17 mL dissolved, remainder from hemoglobin).
- Oxygen loading in lungs per 100 mL blood: whole blood (19.8 mL), hemoglobin solution (19.5 mL), plasma (0.29 mL).
- In venous blood at P_O2 = 40 mm Hg: whole blood (15.2 mL), hemoglobin solution (15.1 mL), plasma (0.12 mL).
- Approximately 5 mL O₂ is transported per 100 mL blood from lungs to tissues.
Respiratory Exchange Ratio
- The respiratory exchange ratio (R) is the ratio of CO₂ output to O₂ uptake at any given time, whether or not equilibrium is reached: R = Rate of CO₂ output / Rate of O₂ uptake.
- In steady-state conditions, this ratio is called the respiratory quotient (RQ).
- RQ values:
- Carbohydrate: 1.00.
- Fat: 0.70.
- Protein: 0.82.
- Mixed diet: 0.825.
- Extraction ratio = (Oxygen consumption / Oxygen supply) × 100.
- Arterial blood carries ~20.1 mL O₂/100 mL, and venous blood carries ~15 mL O₂/100 mL, delivering ~5 mL O₂ to tissues.
- Extraction ratio is ~25% at rest but can increase during exercise.
Oxyhemoglobin Dissociation Curve (OHDC)
- The OHDC plots the partial pressure of O₂ against the percentage saturation of hemoglobin with O₂, showing a sigmoid shape due to cooperative binding.
- P50 is the PO2 at which hemoglobin is 50% saturated: 26 mm Hg in arterial blood, 29 mm Hg in venous blood.
- Key points on the OHDC:
- PO2 = 0 mm Hg, SO2 = 0% (origin).
- PO2 = 26 mm Hg, SO2 = 50% (P_50).
- PO2 = 40 mm Hg, SO2 = 75% (normal mixed venous blood).
- PO2 = 95 mm Hg, SO2 = 97% (normal arterial blood).
- PO2 = 100 mm Hg, SO2 = 98% (nearly fully saturated arterial blood).
- A right shift in the OHDC indicates reduced hemoglobin affinity for O₂, favoring O₂ delivery and increasing P_50.
Factors Causing Displacement Of Oxyhemoglobin Dissociation Curve (OHDC)
Bohr Effect:
- Changes in blood hydrogen ions or CO₂ affect hemoglobin’s O₂ affinity.
- Decreased pH (acidosis) reduces O₂ affinity, causing a rightward shift of the OHDC.
Effect of pH:
- Decreased pH: Direct right shift; indirectly left shift by decreasing 2,3-DPG.
- Increased pH: Direct left shift; indirectly right shift by increasing 2,3-DPG.
Temperature:
- Shifts OHDC left in hypothermia and right in hyperthermia.
- Increased body temperature during exercise contributes to a right shift, alongside increased 2,3-DPG.
2,3-Diphosphoglycerate (2,3-DPG):
- One 2,3-DPG molecule binds between hemoglobin’s β-chains, stabilizing the T conformation, reducing O₂ affinity, and shifting OHDC right.
- Formed in the Rapoport-Luebering shunt of glycolysis, with levels determined by synthesis (DPG mutase) and degradation (DPG phosphatase).
- High pH (alkalosis) enhances DPG mutase and reduces DPG phosphatase, increasing 2,3-DPG levels.
DPG Level with Blood Storage:
- Storage at <6°C reduces glycolysis to <5% of normal, depleting 2,3-DPG to near zero within 1-2 weeks, shifting OHDC left.
- Modern preservation solutions (e.g., citrate-phosphate-dextrose) with dextrose, citrate, and adenine/phosphate slow DPG depletion but levels still become negligible within 2 weeks.
- Post-transfusion, DPG levels reach ~50% of normal within 7 hours and fully recover within 48 hours.
- Anemia, high altitude, and other factors increase 2,3-DPG levels.
Abnormal Forms of Hemoglobin:
- Fetal hemoglobin (HbF): Composed of two γ-chains and two α-chains, with lower affinity for 2,3-DPG, causing a left shift (P_50 = 19 mm Hg vs. 26 mm Hg for HbA).
- Sickle cell anemia: Causes a right shift of OHDC.
- Thalassemia: Causes a left shift of OHDC.
- Methemoglobin: Oxidized iron (ferric) increases O₂ affinity in remaining ferrous heme sites, reducing O₂ release and shifting OHDC left.
Carboxyhemoglobin (HbCO):
- CO binds hemoglobin 250 times more strongly than O₂, though the combination rate is slower.
- CO binding is slower when displacing O₂ from HbO₂: CO + HbO₂ → HbCO + O₂.
- High oxygen saturation reduces CO binding rate, utilized in hyperbaric oxygen therapy for CO poisoning.
- CO reduces hemoglobin’s O₂ carrying capacity, decreases P50, and shifts the OHDC left, partly due to reduced 2,3-DPG.
- CO affects cellular cytochromes only at 1000 times the lethal dose, so tissue toxicity is not a factor in clinical CO poisoning.
Myoglobin:
- Present in high amounts in muscles for sustained contraction (e.g., leg and heart muscles).
- Binds one O₂ molecule per molecule, taking up O₂ readily at low pressure (95% saturated at PO2 = 40 mm Hg vs. 75% for hemoglobin).
- The oxygen-myoglobin dissociation curve (OMDC) is a rectangular hyperbola, lying left of the OHDC.
Factors Causing OHDC Shifts:
- Right Shift:
- Decreased pH, increased CO₂, increased temperature, increased 2,3-DPG, sickle hemoglobin.
- Increased 2,3-DPG caused by: exercise, high altitude, anemia, alkalosis, inosine, pyruvate, phosphate, thyroid hormones, growth hormone, androgens, hypoxia.
- Left Shift:
- Stored blood, fetal hemoglobin (γ-chains don’t bind 2,3-DPG), CO poisoning (partial HbCO saturation), hypothermia, alkalosis, hypocapnia, thalassemia, methemoglobin, cyanide, nitric acid.
Co₂ Transport
- Arterial blood contains ~48 mL/dL total CO₂; systemic capillaries add ~4 mL/dL, making mixed venous blood ~52 mL/dL.
- Forms of CO₂ transport:
- Dissolved CO₂: ~10%.
- As bicarbonate (HCO₃⁻): ~68%.
- As carbamino compounds: ~22%.
- Distribution: ~11% of total CO₂ remains in plasma, ~89% enters red blood cells (RBCs).
- Table 10.7: Forms of CO₂ Transport:
- Plasma (~11%): Dissolved CO₂ (5%), carbamino compounds (1%), HCO₃⁻ (5%).
- RBC (~89%): Dissolved CO₂ (5%), carbamino compounds (21%), HCO₃⁻ (63%).
- Total: Dissolved CO₂ (10%), carbamino compounds (22%), HCO₃⁻ (68%).
- Carbamino Compounds:
- In plasma: CO₂ combines with plasma proteins.
- In RBCs: CO₂ combines with hemoglobin (Hb).
- More carbamino compounds form in RBCs due to higher hemoglobin concentration (33 g/dL) compared to plasma proteins (8 g/dL) and hemoglobin’s greater ease of forming carbamino compounds.
- Bicarbonate (HCO₃⁻):
- CO₂ diffuses into plasma, dissolves, and reacts with water to form carbonic acid (H₂CO₃), which dissociates into H⁺ and HCO₃⁻: CO₂ + H₂O ↔ H₂CO₃ ↔ H⁺ + HCO₃⁻.
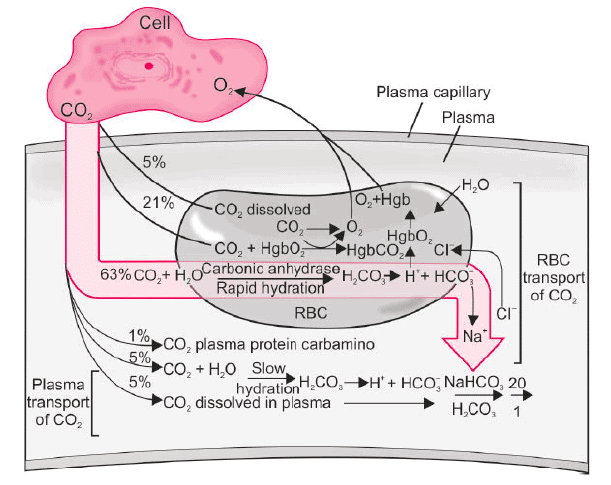
- This reaction is slow but accelerated 5000 times by carbonic anhydrase (CA) in RBCs.
- HCO₃⁻ diffuses out of RBCs in exchange for Cl⁻ via the anion exchanger (AE1), a process called the chloride shift or Hamburger shift.
- Free H⁺ is buffered by hemoglobin, driving HCO₃⁻ synthesis.
- CO₂ diffuses into plasma, dissolves, and reacts with water to form carbonic acid (H₂CO₃), which dissociates into H⁺ and HCO₃⁻: CO₂ + H₂O ↔ H₂CO₃ ↔ H⁺ + HCO₃⁻.
- In pulmonary capillaries, CO₂ moves from RBCs and plasma into alveolar air, reversing the above reactions; Cl⁻ and H₂O leave RBCs, causing them to shrink.
- Haldane Effect:
- Oxygen binding to hemoglobin in pulmonary capillaries displaces CO₂ from blood, enhancing CO₂ transport.
- Oxyhemoglobin is a stronger acid, reducing hemoglobin’s tendency to form carbaminohemoglobin and releasing H⁺ ions.
- Released H⁺ binds HCO₃⁻ to form H₂CO₃, which dissociates into H₂O and CO₂, released into alveoli.
- The Haldane effect is more significant for CO₂ transport than the Bohr effect is for O₂ transport.
- Significance of Haldane Effect:
- Figure 10.9 shows CO₂ dissociation curves at P_O2 = 100 mm Hg (lungs) and 40 mm Hg (tissues).
- At tissues (Point A): PCO2 = 45 mm Hg, CO₂ content = 52 vol%.
- At lungs (Point B): Haldane effect shifts the curve, reducing CO₂ content to 48 vol% at PCO2 = 40 mm Hg, losing 4 vol% CO₂.
- Without Haldane effect (Point C), CO₂ content would drop to 50 vol%, losing only 2 vol%.
- The Haldane effect doubles CO₂ release in lungs and CO₂ pickup in tissues.
Hypoxia
- Hypoxia: Oxygen deficiency at the tissue level.
- Hypoxemia: Decreased partial pressure of oxygen (PaO₂) in arterial blood.
Four Categories of Hypoxia (Table 10.8):
- Hypoxic Hypoxia:
- Reduced arterial PO2.
- Causes: High altitude, pneumonia, pulmonary fibrosis, ventilation-perfusion imbalance, venous-to-arterial shunts.
- Features: Low PaO₂, low PvO₂, decreased (a-v) PO2, decreased dissolved and Hb-bound O₂, stimulates peripheral and central chemoreceptors (if high CO₂), cyanosis present.
- Anemic Hypoxia:
- Normal arterial PO2 but reduced hemoglobin available for O₂ transport.
- Causes: Anemia, CO poisoning, methemoglobinemia.
- Features: Normal PaO₂, low PvO₂, increased (a-v) PO2, normal dissolved O₂, decreased Hb-bound O₂, peripheral chemoreceptors not stimulated, central chemoreceptors not stimulated, cyanosis rare.
- Stagnant/Ischemic Hypoxia:
- Inadequate blood flow to tissues despite normal PO2 and hemoglobin.
- Causes: Slow circulation (heart failure), shock, hemorrhage.
- Features: Normal PaO₂, low PvO₂, increased (a-v) PO2, normal dissolved and Hb-bound O₂, peripheral chemoreceptors stimulated, central chemoreceptors not stimulated, cyanosis present.
- Histotoxic Hypoxia:
- Adequate O₂ delivery but tissues cannot utilize O₂ due to toxic agents.
- Causes: Cyanide poisoning.
- Features: Normal PaO₂, high PvO₂, decreased (a-v) P_O2, normal dissolved and Hb-bound O₂, peripheral chemoreceptors stimulated, central chemoreceptors not stimulated, cyanosis never present.
- Hypoxic hypoxia is the most common clinical form, often caused by ventilation-perfusion mismatch.
- Arterial-venous (a-v) oxygen difference increases in stagnant hypoxia (diagnostic) but decreases in histotoxic hypoxia (diagnostic).
- Peripheral chemoreceptors have high blood flow (2000 mL/100 g/min), meeting O₂ needs via dissolved O₂, so they are not stimulated in anemia or CO poisoning.
Cyanosis
- Cyanosis is a bluish discoloration of mucous membranes and/or skin.
- Cyanosis detection thresholds in capillary blood:
- Deoxyhemoglobin: ≥4 g/dL.
- Methemoglobin: ≥ 1.5 g/dL.
- Sulfhemoglobin: ≥ 0.5 g/dL.
- Central cyanosis may be detectable at SaO₂ of 85%, but in dark-skinned individuals, it may require SaO₂ ≤75%.
Oxygen Treatment Of Hypoxia
- Oxygen therapy is ineffective for stagnant, anemic, histotoxic hypoxia, and hypoxic hypoxia due to shunting of unoxygenated venous blood past the lungs.
- Oxygen therapy is highly beneficial for other forms of hypoxic hypoxia.
- Hyperbaric O₂ Therapy:
- Effective for: CO poisoning, radiation-induced tissue injury, gas gangrene, severe blood loss anemia, diabetic leg ulcers, slow-healing wounds, and rescue of skin flaps/grafts with marginal circulation.
- Primary treatment for decompression sickness and air embolism.
- Neurons are most sensitive to hypoxia, followed by oligodendrocytes, astrocytes, and endothelium.
- Most sensitive neurons: Pyramidal cells of CA1 hippocampus (Sommer’s sector), Purkinje cells of cerebellum, striatal neurons.
- Most resistant to hypoxia: Brain stem and spinal cord.
- Gray matter is more sensitive than white matter.
- Irreversible damage occurs if no O₂ supply for >5 minutes.
|
40 docs|9 tests
|
FAQs on Gaseous Exchange Chapter Notes - Physiology - NEET PG
| 1. What is the difference between perfusion-limited and diffusion-limited gas exchange? |  |
| 2. How is the diffusing capacity of the respiratory membrane measured? |  |
| 3. What are the primary mechanisms of oxygen transport in the blood? |  |
| 4. What is the significance of the oxyhemoglobin dissociation curve (OHDC)? |  |
| 5. What are the different ways carbon dioxide is transported in the blood? |  |





















