NEET PG Exam > NEET PG Notes > Physiology > Chapter Notes: Male and Female Reproductive Physiology
Male and Female Reproductive Physiology Chapter Notes | Physiology - NEET PG PDF Download
| Table of contents |

|
| Male Reproductive Physiology |

|
| Ovarian Steroids: Estrogen and Progesterone |

|
| Inhibins and Activins |

|
| Relaxin: A Pleiotropic Hormone |

|
| Puberty |

|
Male Reproductive Physiology
Regulation of Testes
- Hypothalamic control involves gonadotropin-releasing hormone (GnRH) secreted by arcuate nuclei of the hypothalamus into the hypothalamic-hypophysial portal blood.
- GnRH stimulates the anterior pituitary to secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
- FSH acts on Sertoli cells to maintain spermatogenesis and prompts Sertoli cells to secrete inhibin, which negatively feeds back to regulate FSH secretion.
- LH acts on Leydig cells to promote testosterone synthesis.
- Testosterone reinforces spermatogenic effects of FSH in Sertoli cells via an intratesticular paracrine mechanism.
- Testosterone inhibits LH secretion by reducing GnRH release from the hypothalamus and directly inhibiting LH release from the anterior pituitary.
- Inhibin, produced by Sertoli cells, inhibits FSH secretion from the anterior pituitary.
- 5α-reductase inhibitors (e.g., finasteride) are used to treat benign prostatic hyperplasia by blocking the conversion of testosterone to dihydrotestosterone in the prostate.
Synthesis of Testosterone
- Testosterone is the primary androgen synthesized and secreted by Leydig cells.
Spermatogenesis
- Spermatogenesis is the process of male gamete development from primordial germ cells through spermatogonia, spermatocytes, and spermatids to mature haploid spermatozoa.
- It begins during adolescence.
- A single spermatogonium can produce approximately 512 spermatids.
- In humans, it takes an average of 74 days to form a mature sperm from a primitive germ cell.
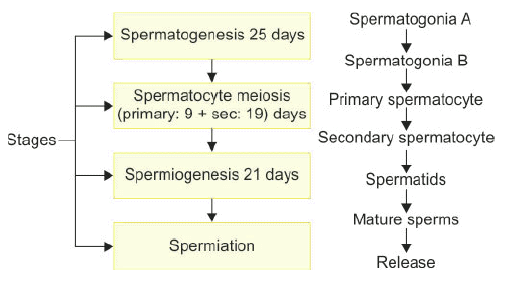 Steps in spermatogenesis.
Steps in spermatogenesis. - Testosterone is critical for spermiogenesis and spermiation (release of mature sperm).
- FSH supports Sertoli cell proliferation up to the pachytene spermatocyte stage and is essential for spermatogenesis and meiosis.
Role of Estrogen in Male Reproduction
- Estrogen levels in rete testis fluid are very high.
- Estrogen facilitates fluid resorption and spermatozoa concentration; failure leads to diluted sperm in the epididymis, causing infertility.
- Estrogen is involved in acrosome biogenesis.
- Estrogen supports spermiation by aiding cytoskeletal organization of proteins necessary for sperm release, alongside testosterone, which is needed for ectoplasmic specialization formation.
Changes in Sperm inside Epididymis
- Spermatozoa leaving the testes are not fully mobile.
- They acquire forward progressive motility during passage through the epididymis.
- Motility involves activation of CatSper, a unique Ca²⁺ ion channel in the sperm tail that permits cAMP-mediated Ca²⁺ influx.
- Epididymal transit time ranges from 12 to 26 days.
- Sperm maturation in the epididymis enables fertilization, as evidenced by pregnancy results after micro-epididymal sperm aspiration (MESA).
- Sperm gain progressive forward motility and undergo acrosome maturation.
- There is a decrease in cytoplasm and cell volume.
- Sperm develop the ability to bind to the zona pellucida by gaining specific receptors.
- Molecular reorganization of the plasma membrane occurs, including lipid stabilization and protein shedding/acquisition.
Capacitation and Acrosomal Reaction
- Capacitation occurs in the female genital tract (from cervix to isthmus of the fallopian tube), enhancing sperm hypermotility for fertilization.
- Capacitation transforms sperm from non-fertilizing to potentially fertilizing cells through complex changes.
- Capacitated sperm respond to molecular signals from the oocyte, cumulus cells, and zona pellucida, undergoing the acrosome reaction.
- Capacitation involves cholesterol efflux from the sperm plasma membrane, increased membrane fluidity, modulations in intracellular ion concentrations (Ca²⁺, K⁺, Na⁺, HCO₃⁻), plasma membrane hyperpolarization, and increased protein tyrosine phosphorylation.
- Capacitation starts at the cervix and is completed in the isthmus.
- The acrosome reaction, essential for zona pellucida binding and penetration, occurs in the ampulla of the fallopian tube.
- The acrosome reaction is an irreversible exocytosis event releasing hydrolytic enzymes to aid zona pellucida penetration and modifying the sperm head plasma membrane for oocyte fusion.
- Sperm speed is approximately 3 mm/min through the female genital tract, reaching the uterine tubes 30–60 minutes after copulation.
Semen
- Semen comprises sperm and secretions from seminal vesicles, prostate, Cowper’s glands, and possibly urethral glands.
- Average ejaculate volume is 2.5–3.5 mL after several days of abstinence.
- Semen composition includes specific gravity of 1.028, pH of 7.35–7.50, and an average sperm count of about 100 million/mL.
- Seminal vesicles contribute 60% of semen volume, including fructose, phosphorylcholine, ergothioneine, ascorbic acid, flavins, and prostaglandins.
- Prostate contributes 20% of semen volume, including spermine, citric acid, cholesterol, phospholipids, fibrinolysin, fibrinogenase, zinc, and acid phosphatase.
- Semen contains phosphate bicarbonate buffers and hyaluronidase but lacks thrombin or prothrombin.
Coagulation and Liquefaction of Semen
- Semen coagulates soon after ejaculation due to clotting factors from seminal vesicles, forming a gelatinous mass with semenogelin as the major protein.
- Coagulated semen liquefies after 15–60 minutes due to prostatic proteolytic enzymes, including seminin, plasminogen activators, and prostate-specific antigen (PSA).
- Coagulation coats sperm with nutrients and factors aiding fertilization (e.g., capacitation factor) and facilitates sperm deposition near the vaginal os.
- Liquefaction allows sperm to swim out of the coagulum into the cervix and uterus.
- Semen coagulation is unique, not involving prothrombin, fibrinogen, or hematologic clotting factors, and is not inhibited by sodium citrate or heparin.
- Main fibrous coagulum proteins are semenogelin I and semenogelin II, produced by seminal vesicles.
Female Reproductive Physiology
Menstrual Cycle
- Unlike other mammals with an estrous cycle, primates (including humans) have a menstrual cycle with regular cyclic changes.
- The average menstrual cycle length is 28 days, with day 1 defined as the first day of menstrual bleeding.
- The menstrual cycle includes ovarian and uterine (endometrial) cycles, divided into phases: follicular phase (days 1–14, including menstrual and proliferative phases), ovulation (day 14), and luteal phase (days 15–28, secretory phase).
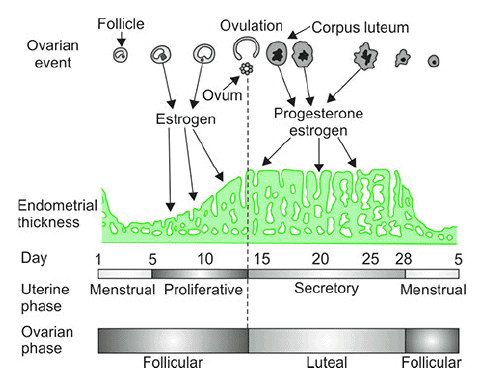 The endometrial (uterine) cycle and ovarian cycle.
The endometrial (uterine) cycle and ovarian cycle. - Menstrual phase (days 1–5): Withdrawal of hormonal support causes endometrial necrosis and menstruation, with low estrogen and progesterone levels.
- Proliferative phase (days 6–14): Estrogen dominates, promoting replacement of endometrial cells lost during menses.
- Ovulation (day 14): Characterized by an LH surge that induces ovulation.
- Secretory phase (days 15–28): Progesterone, along with estrogen, prepares the uterus for implantation.
Ovarian Cycle
- From birth, ovaries contain many primordial follicles, each with an immature ovum.
- During the follicular phase, primordial follicles develop into mature Graafian follicles under FSH influence.
- Follicular development includes preantral (primordial, primary, secondary, tertiary) and antral (Graafian, preovulatory) follicles.
- Preantral follicle development is gonadotropin-independent, while antral follicle development is gonadotropin-dependent.
- Oogenesis is the differentiation of primitive germ cells into mature ova, starting during early embryonic development.
- Oogonia proliferate by mitosis to form primary oocytes, which develop into primordial follicles.
- Meiosis in females begins in mid-gestational fetal life, unlike in males, where it starts at puberty.
- The Graafian follicle’s granulosa cells are the primary source of circulating estrogen, with theca interna cells aiding estrogen production.
- Just before ovulation, the Graafian follicle reaches approximately 20 mm in size.
- First polar body extrusion occurs during telophase I of the first meiotic division, 20 hours after the LH surge onset, signaling oocyte nuclear maturity.
- After first polar body extrusion, the second meiotic division begins, arresting at metaphase II about 3 hours before ovulation.
- Ovulation involves follicle rupture and ovum extrusion into the abdominal cavity, occurring 24–36 hours after LH surge onset, 9–12 hours after LH peak, 83 hours after estradiol rise, or 24–36 hours after estradiol peak.
- The ruptured follicle fills with blood (corpus hemorrhagicum), converting into the yellowish, lipid-rich corpus luteum, initiating the luteal phase with estrogen and progesterone secretion.
- If pregnancy occurs, the corpus luteum persists; otherwise, it degenerates around day 24, forming a corpus albicans.
- Minor bleeding from the ruptured follicle may cause peritoneal irritation and fleeting lower abdominal pain (mittelschmerz).
Hormonal Control of Female Sexual Cycle
- Estrogens switch from negative to positive feedback when levels rise above a threshold for at least two days, triggering an LH and FSH surge, with the LH surge essential for ovulation and corpus luteum formation.
- In the luteal phase, the endometrium becomes vascularized and edematous under estrogen and progesterone influence.
- Corpus luteum luteal cells, stimulated by LH, secrete progesterone and some estrogen, with progesterone inhibiting LH secretion.
- Progesterone causes endometrial swelling, secretory development, coiled uterine glands, increased stromal cell cytoplasm, lipid/glycogen deposits, and enhanced endometrial blood supply.
- Progesterone from the corpus luteum inhibits LH, contributing to corpus luteum regression if pregnancy does not occur.
- Corpus luteum regression lowers progesterone and estradiol levels, leading to endometrial necrosis, spiral artery spasms, and menstrual flow.
- At menstruation’s end, all but the deep endometrial layers are sloughed.
- Menstrual blood is primarily arterial (75%), contains tissue debris, prostaglandins, and fibrinolysin, lasts 3–5 days, and averages 30 mL blood loss (abnormal if >80 mL).
- Pulsatile GnRH release (every 60–90 minutes) from the hypothalamus triggers LH and FSH secretion from the anterior pituitary.
- Theca cells have LH receptors, while granulosa cells have both LH and FSH receptors.
- Ovarian steroids (estrogens, progestins) exert negative feedback on the hypothalamic-pituitary axis during most of the cycle, reducing LH and FSH release.
- At the follicular phase’s end, high estradiol levels for at least 2 days switch to positive feedback, promoting the LH surge for ovulation and luteinization.
- Post-ovulation, LH and FSH levels drop due to negative feedback from estradiol, progesterone, and inhibin.
- During the luteal phase, luteal cells increase progesterone, estradiol, and inhibin synthesis.
- By 48 hours before menses, LH pulse frequency decreases to one every 3–4 hours, reducing LH levels.
- Late luteal phase corpus luteum demise lowers progesterone, estradiol, and inhibin, shifting to a follicular-phase LH secretion pattern post-menstruation.
- FSH is slightly elevated in the follicular phase, increasing estrogen secretion from granulosa cells.
- Midcycle LH and FSH peaks result from estrogen’s positive feedback.
- Inhibins inhibit FSH secretion, while activins stimulate it independently of GnRH.
- High-frequency, low-amplitude GnRH in the follicular phase favors LH synthesis, while slow-frequency, high-amplitude GnRH in the luteal phase favors FSH synthesis.
- Sex hormone-binding globulin (SHBG), also known as testosterone-binding globulin (TBG), binds estradiol and is twice as high in women, with estrogens (including birth control pills) stimulating its synthesis.
Ovarian Steroids: Estrogen and Progesterone
- The ovary synthesizes estradiol (major estrogen) and progesterone (major progestin) from cholesterol.
- Estrogen biosynthesis requires theca and granulosa cells and LH and FSH, while progesterone synthesis needs only one cell type.
- Theca cells produce adrenal androgens but lack aromatase for estrogen production.
- Granulosa cells have aromatase but lack 17α-hydroxylase and 17,20-desmolase for androgen production.
- Theca cells, near blood vessels, source LDL cholesterol, while granulosa cells, surrounded by LDL-poor follicular fluid, synthesize cholesterol de novo in the follicular phase.
- Post-ovulation, corpus luteum vascularization allows granulosa-lutein cells to uptake LDL cholesterol for progesterone synthesis.
- Theca cells have LH receptors; granulosa cells have both LH and FSH receptors.
- The two-cell, two-gonadotropin hypothesis outlines estrogen and progesterone synthesis:
- LH primes theca cells to convert cholesterol to androstenedione, which diffuses to granulosa cells.
- FSH-stimulated granulosa cell aromatase converts androstenedione to estradiol.
- Post-ovulation, LH acts on corpus luteum cells, with vascularization enabling both theca and granulosa cells to produce progesterone.
- Some progesterone diffuses to theca cells for 17α-hydroxyprogesterone synthesis.
- Theca cells produce androstenedione, which granulosa cells convert to estradiol.
- Estradiol secretion rates: 36 μg/day (early follicular), 380 μg/day (pre-ovulation), 250 μg/day (midluteal); in men, ~50 μg/day.
- Progesterone levels: ~0.3 ng/mL in men; ~0.9 ng/mL in women during the follicular phase.
- Estrogen forms: 17β-estradiol (E2), estrone (E1), estriol (E3); 2% free, 60% albumin-bound, 38% SHBG-bound.
- Progesterone: C21 steroid; 2% free, 80% albumin-bound, 18% corticosteroid-binding globulin-bound.
- Estrogen metabolism: E1, E2, E3 converted to glucuronide/sulfate conjugates in the liver, excreted in urine.
- Progesterone metabolism: Converted to pregnanediol in the liver, conjugated to glucuronic acid, excreted in urine.
- Estrogen receptors: Intranuclear (ERα/β).
- Progesterone receptors: Intracytoplasmic (PR-B), intranuclear (PR-A).
- Estrogen effects on female genitalia: Promotes ovarian follicle growth, endometrial proliferation, increased uterine tube motility, decreased secretion, enhanced uterine smooth muscle excitability, and copious, elastic cervical mucus (spinbarkeit).
- Progesterone effects on female genitalia: Induces secretory endometrial changes (tortuous glands), decreases uterine tube motility, increases secretion, reduces uterine smooth muscle excitability, increases resting membrane potential, and produces scanty, thick cervical mucus.
- Estrogen effects on secondary sex characteristics: Enlarges breasts (ducts and stroma), uterus, vagina, and determines female fat distribution.
- Progesterone effects on secondary sex characteristics: Enlarges breasts (lobules and alveoli).
- Estrogen endocrine effects: Increases angiotensinogen and thyroid-binding globulin secretion, causes epiphyseal closure.
- Progesterone endocrine effects: Large doses inhibit LH secretion, potentiate estrogen’s ovulation inhibition.
- Estrogen CNS effects: Increases libido via hypothalamic action, promotes dendrite proliferation, synaptic knob formation, slightly increases basal metabolic rate (BMR).
- Progesterone CNS effects: Thermogenic, responsible for basal body temperature rise at ovulation.
- Other estrogen effects: Salt/water retention, fluidic sebaceous gland secretion (prevents acne), lowers plasma cholesterol (decreases LDL, increases HDL), vasodilation via increased nitric oxide production.
- Other progesterone effects: Natriuresis by blocking aldosterone, stimulates respiration (decreases alveolar PCO₂), increases plasma LDL, decreases HDL.
- Estrogen determines the flat-topped female pubic hair pattern (escutcheon), while androgens drive pubic and axillary hair growth in both sexes.
- Gonadal steroids (testosterone in boys, estradiol in girls) are major hormones for bone growth and osseous maturation.
Inhibins and Activins
- Inhibins are produced by granulosa cells, pituitary, brain, adrenal gland, kidney, bone marrow, corpus luteum, and placenta.
- FSH specifically stimulates granulosa cells to produce inhibins, which inhibit FSH production via negative feedback.
- Estradiol may stimulate inhibin production via an intraovarian mechanism.
- Pre-ovulation, LH stimulates inhibin production by granulosa cells after they acquire LH receptors.
- Activins, produced in the same tissues, stimulate FSH release from pituitary cells.
- Inhibins are dimeric glycoproteins with an α subunit linked to a β_A (inhibin A) or β_B (inhibin B) subunit via disulfide bonds.
- Inhibin A predominates in late follicular and luteal phases; inhibin B predominates in early and mid-follicular phases.
- Inhibin A is mainly secreted by the corpus luteum; inhibin B by granulosa cells.
Relaxin: A Pleiotropic Hormone
- Relaxin, a ~6000 Da peptide hormone in the insulin family, has two disulfide-linked chains (A and B), with the B chain bearing the receptor interaction site.
- It reaches peak plasma levels during pregnancy, produced by the decidua, placenta, and breast.
- In nonpregnant women, relaxin is produced by the corpus luteum and endometrium during the secretory phase, not the proliferative phase.
- In males, relaxin is synthesized in the prostate and released in seminal fluid.
- The heart atria are an additional relaxin source.
- Functions include collagen remodeling for birth canal softening, inhibition of uterine contractile activity, and stimulation of mammary gland growth and differentiation.
Puberty
- Prepubertal stage (early childhood to 8–9 years): Undetectable serum LH and sex hormones (estradiol in girls, testosterone in boys).
- 1–3 years before puberty: Low serum LH levels during sleep, reflecting pulsatile hypothalamic GnRH discharge.
- Nocturnal LH pulses increase in amplitude and frequency as puberty approaches; by midpuberty, LH pulses occur every 90–120 minutes, including daytime.
- Pulsatile hypothalamic GnRH secretion drives pubertal development.
- KISS1R (GPR54) gene mutations: Loss-of-function causes hypogonadotropic hypogonadism; gain-of-function causes precocious puberty.
- Estrogens, not androgens, drive bone maturation, epiphyseal fusion, and growth cessation.
- A large nocturnal gonadotropin increase is a puberty hallmark.
- In pubertal boys, nocturnal testosterone rise parallels gonadotropin elevation; in girls, higher estradiol levels occur daytime.
- GnRH pulse generator is regulated by stimulatory (glutamic acid, kisspeptin, neurokinin-B) and inhibitory (GABA, preproenkephalin, dynorphin) neuropeptides, and glial cell factors like transforming growth factor α.
- Aromatization of testosterone to estrogen at the growth plate is required for growth plate fusion.
- Estrogens mediate bone elongation acceleration, epiphyseal closure, peak bone mass achievement, and bone mass maintenance.
- Estrogens and growth hormone, along with direct sex steroid effects, drive the pubertal growth spurt.
- GnRH is the major hormone for puberty onset and progression, requiring pulsatile (not continuous) release.
- Gonadal steroids (testosterone in boys, estradiol in girls) are critical for bone growth and osseous maturation.
The document Male and Female Reproductive Physiology Chapter Notes | Physiology - NEET PG is a part of the NEET PG Course Physiology.
All you need of NEET PG at this link: NEET PG
|
40 docs|9 tests
|
FAQs on Male and Female Reproductive Physiology Chapter Notes - Physiology - NEET PG
| 1. What are the primary functions of estrogen and progesterone in the female reproductive system? |  |
Ans. Estrogen and progesterone are essential ovarian steroids that play crucial roles in the female reproductive system. Estrogen is primarily responsible for the development of secondary sexual characteristics, regulation of the menstrual cycle, and preparation of the uterus for implantation. Progesterone, on the other hand, is important for maintaining the uterine lining after ovulation and supporting early pregnancy by preventing uterine contractions.
| 2. How do inhibins and activins regulate reproductive hormones? |  |
Ans. Inhibins and activins are glycoproteins that play a significant role in the regulation of reproductive hormones. Inhibins primarily inhibit the secretion of follicle-stimulating hormone (FSH) from the anterior pituitary gland, thereby regulating the growth and maturation of ovarian follicles. Activins, in contrast, stimulate FSH secretion and promote follicular development. Together, these hormones help maintain the balance of reproductive hormone levels during the menstrual cycle.
| 3. What is relaxin and what roles does it play in the reproductive system? |  |
Ans. Relaxin is a pleiotropic hormone produced primarily by the ovaries and placenta during pregnancy. It has several roles, including relaxing the uterine muscles, which helps accommodate a growing fetus, and softening the cervix in preparation for childbirth. Additionally, relaxin is involved in remodeling connective tissues and may have effects on the cardiovascular system during pregnancy.
| 4. How does puberty affect male reproductive physiology? |  |
Ans. Puberty marks a critical transition in male reproductive physiology characterized by increased production of testosterone and other hormones. This leads to the development of secondary sexual characteristics such as increased muscle mass, voice deepening, and the growth of facial and body hair. Additionally, puberty initiates the maturation of the testes and the beginning of sperm production, enabling fertility.
| 5. What are the implications of hormonal imbalances in male and female reproductive health? |  |
Ans. Hormonal imbalances in reproductive health can lead to various issues in both males and females. In females, imbalances in estrogen and progesterone can cause irregular menstrual cycles, polycystic ovary syndrome (PCOS), or endometriosis. In males, low testosterone levels can result in decreased libido, infertility, and other health issues. Proper understanding and management of these hormonal levels are essential for maintaining reproductive health.
Related Searches




















