Metals and Non-metals Chapter Notes | Chemistry Class 7 ICSE PDF Download
| Table of contents |

|
| Introduction |

|
| Metals |

|
| Non-Metals |

|
| Metalloids |

|
Introduction
We know of 118 chemical elements, each with unique traits and some shared features. Based on their characteristics, these elements are grouped into two main categories: metals and non-metals.Certain elements exhibit traits of both metals and non-metals and are called metalloids. Examples include arsenic, antimony, and silicon.
Metals are essential in our everyday lives. They are used to make cooking pots, doors, windows, gates, electric fans, sewing machines, washing
machines, cars, buses, trucks, trains, ships, and aeroplanes. These are often made from metals or metal mixtures known as alloys.
Non-metals are also crucial for daily life. Life on Earth depends on non-metals, as they are vital for existence. Carbon, a key non-metal, forms compounds such as carbohydrates, fats, and proteins that support life. These compounds, along with vitamins and enzymes, are necessary for the growth and development of living organisms. Oxygen, another non-metal, is essential for survival and for the burning of fuels that give us energy for various needs. Nitrogen, found in the air, slows down burning to make it safer. Sulphur is present in many plant and animal substances. Non-metals are also used to produce vegetable ghee, fertilisers, acids, explosives, fungicides, and more.
Metals
Metals are elements that lose electrons to form positive ions called cations.Occurrence of Metals
Metals exist in nature in two forms: free state and combined state.- Free state: Less reactive metals like gold and platinum are found naturally in pure form. Copper and silver can be found in both free and combined states.
- Combined state: Most metals are reactive and found as minerals (e.g., metal oxides, carbonates, sulphides, chlorides).
- A mineral is a naturally occurring solid with a definite chemical composition and crystalline structure.
- The earth’s crust is the main source of metals, with aluminium being the most abundant, followed by iron and calcium.
- Extracting pure metals from minerals involves several steps, but it’s not always economical.
Properties of Metals
- State: Most metals are solid and hard at room temperature. Exceptions: sodium and potassium are soft; mercury and gallium are liquid.
- Lustre: Metals are shiny. Gold has yellow lustre, copper is reddish-brown, and iron, silver, aluminium, and magnesium have white lustre.
- Density: Metals are heavy due to high density. Exceptions: lithium, sodium, and potassium have low density and float on water.
- Malleability: Metals can be beaten into thin sheets. Gold and silver are highly malleable. Exception: zinc is brittle and breaks when hammered.
- Ductility: Metals can be drawn into wires. Gold and silver are highly ductile. Exception: zinc is brittle.
- Melting and Boiling Points: Metals have high melting and boiling points.
- Thermal and Electrical Conductivity: Metals conduct heat and electricity well. Silver is the best conductor, followed by copper and aluminium. Exceptions: lead and tungsten are poor conductors.
- Sonority: Metals produce a ringing sound when struck.
- Tensile Strength: Metals can withstand a lot of strain. Iron has high tensile strength. Exception: zinc has low tensile strength.
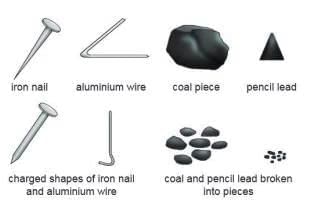
Activity 6.1
Aim:To show that metals are malleable.?Materials Required: Iron nail, coal piece, aluminium wire, pencil lead, hammer.
Procedure: Beat each material with a hammer.
Observation: Iron nail and aluminium wire flatten and change shape; coal and pencil lead break into pieces.
Conclusion: Metals (iron, aluminium) are malleable; non-metals (coal, pencil lead) are not.
Activity 6.2
Aim:To show that metals are lustrous.Materials Required: Copper piece, sulphur piece, watch glasses.
Procedure: Observe copper and sulphur in watch glasses.
Observation: Copper shines; sulphur does not.
Conclusion: Metals (copper) are lustrous; non-metals (sulphur) are not.
Activity 6.3
Aim:To show that metals are sonorous.Materials Required: Copper spoon, coal piece.
Procedure: Drop each item on the floor.
Observation: Copper spoon produces a ringing sound; coal does not.
Conclusion: Metals (copper) are sonorous; non-metals (coal) are not.
Activity 6.4
Aim:To show that metals are hard.Materials Required: Iron, copper, aluminium, magnesium pieces, knife.
Procedure: Try cutting each metal with a knife.
Observation: Metals cannot be cut with a knife.
Conclusion: Metals are hard.
Noble Metals
- Noble metals (e.g., gold, silver, platinum) do not react with non-metals.
- They resist corrosion and oxidation in the atmosphere.
Activity 6.5
Aim:To show that metals conduct electricity.Materials Required: Electric circuit with a bulb, cell, iron nail, metal paper clip, coal piece, plastic spoon.
Procedure: Connect each item to the circuit and check if the bulb glows.
Observation: Bulb glows with iron nail and metal paper clip; it does not glow with coal or plastic spoon.
Conclusion: Metals conduct electricity; non-metals and plastics do not.
Corrosion in Metals
- Corrosion happens when a reactive metal gets attacked by moisture in the air.
- The metal reacts with oxygen and water vapor in the air to form new compounds on its surface.
- These compounds make the metal surface look dull, which is called tarnishing.
- The compounds on the surface are usually porous, meaning they have tiny holes, and they slowly fall off.
- When these compounds fall off, the metal underneath gets exposed to more air and water.
- The process of air and water reacting with the metal continues, slowly eating up the entire metal.
- This process of metals getting eaten up by air, moisture, or chemicals like acids is called corrosion.
- In India, the acidic rain with rainwater makes corrosion faster for metals.
- Most metals corrode when they are left in damp air for a long time.
- Corrosion is a big problem because it damages metals and causes a lot of metal to be lost every year.
- The more reactive a metal is, the faster it corrodes.
- We can stop corrosion by using methods like painting, greasing or oiling, galvanising, or electroplating.
Rusting of Iron
- Rusting is the corrosion of iron, forming a red-brown flaky substance called rust (hydrated iron (III) oxide, Fe₂O₃·xH₂O).
- Rusting occurs when iron reacts with oxygen and water in the air.
- Gases like carbon dioxide, sulphur dioxide, sulphur trioxide, and nitrogen dioxide increase the rate of rusting.
Conditions for Rusting
- Rusting of iron happens only when both air and water are present together.
- The speed of rusting increases if certain gases are in the air, such as carbon dioxide, sulphur dioxide, sulphur trioxide, and nitrogen dioxide.
- We can do an activity to prove that both air and water are needed for rusting to happen.
Activity 6.6
Aim:To show that air and water are necessary for rusting.Materials Required: Three test tubes, three iron nails, anhydrous calcium oxide, water, boiled water.
Procedure:
- Place iron nails in three test tubes labeled A, B, and C.
- Test tube A: Add boiled water and seal airtight to remove air.
- Test tube B: Add anhydrous calcium oxide to absorb moisture and seal airtight.
- Test tube C: Add tap water and leave open.
- Observe after a few days.
Observation: Nail in test tube C rusts (exposed to air and water); nails in A (no air) and B (no water) do not rust.
Conclusion: Both air and water are needed for rusting.
Prevention of Rusting
- Iron is a very helpful metal, but it rusts easily when it comes in contact with air and moisture.
- Rusting causes a lot of iron to get wasted, so we need to stop it from happening.
- We can prevent rusting by using different methods, which are explained below.
Oiling/Greasing: Applying oil or grease creates a protective layer on iron tools and machine parts, reducing rust formation and lubricating them.
Painting: Painting of iron articles can be done in different ways."
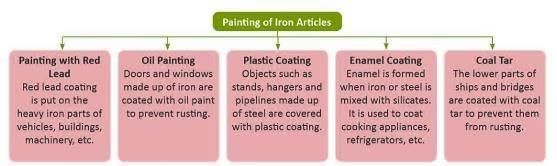
- Galvanisation: Coating iron or steel with zinc to prevent rusting, used for roof sheds, buckets, and water pipes.
- Tinning: Coating iron sheets with tin, a non-toxic and corrosion-resistant metal, for making food cans and boxes.
- Electroplating: Plating iron with metals like chromium, tin, copper, or nickel for durability and decoration (e.g., cutlery, motor parts).
- Chrome Plating: A type of electroplating where chromium is applied to increase corrosion resistance, surface hardness, or for decoration.
- Alloy Formation: Mixing iron with nickel, chromium, or carbon to form stainless steel, which is corrosion-resistant and used for cutlery and surgical instruments.
Activity 6.7
Aim:To show that rust is basic in nature.Materials Required: Rusted iron nail, test tube, water, red and blue litmus papers.
Procedure:
- Crush rust from the nail into a powder.
- Mix the powder with water in a test tube and shake well.
- Test with red and blue litmus papers.
Observation: Blue litmus paper shows no change; red litmus paper turns blue.
Conclusion: Rust is basic in nature.
Uses of Metals
Iron:

- Cheapest and most useful metal, available in three forms based on carbon content.
- Wrought Iron: Purest form (<0.1% carbon), used for tin roofing, railings, furniture, tanks, nails, bolts, gates, and agricultural tools.
- Pig Iron: Impure form (2-4% carbon), used for manhole lids, drain pipes, and immovable machinery parts.
- Steel: Contains up to 2% carbon, used for ships, bridges, buildings, automobiles, and utensils.
Copper:
- Good conductor of heat and electricity, highly ductile and malleable.
- Used for electrical wires, appliances, utensil bases, electronic devices (e.g., computer chips), and alloys like brass and bronze for coins and statues.
Aluminium:
- Silvery-white, ductile, malleable, light, strong, corrosion-resistant, and a good conductor.
- Used for cooking utensils, cans, furniture, door/window frames, electric wires, food packaging (foil), and alloys like duralumin and magnalium for aircraft and automobile bodies.
Zinc:

- Bluish-white, brittle metal.
- Used for dry cells, electrodes, galvanising iron/steel, alloys (brass, bronze, nickelled silver) for utensils, statues, decoration, and extracting gold and silver.
Lead:
- Greyish, ductile, malleable, poor conductor, absorbs radioactive radiation, does not react with dilute acids, steam, or impure water.
- Used for underground cables, pipes, sanitary fittings, bullet tips, tin roofs, nuclear reactor coolants, X-ray screens, alloys (solder, type metal), and car batteries.
Mercury:
- Silvery liquid metal at room temperature.
- Used in thermometers, barometers, sphygmomanometers, scientific apparatus, dental fillings (silver/gold amalgam), and mercury vapour lamps.
Gold:
- Shiny yellow, precious metal.
- Used for coins, jewellery, and dental fillings (gold amalgam).
Fun Fact
- Gold and silver are used for jewellery because they are shiny, malleable, ductile, and corrosion-resistant.
- An alloy of mercury with other metals is called an amalgam.
- ormation.
Silver
- Silver is the best conductor of electricity, very shiny, and highly ductile.
- It is a precious metal used for making electrodes and in electroplating.
- Used to make coins and jewellery.
- Silver amalgam is used for dental fillings.
- Silver nitrate and silver bromide are used in photography.
- Used as a water purifier.
Magnesium
- Magnesium is a silvery-white metal.
- Burns with a dazzling white flame, used in fireworks.
- Used to make alloys for aircraft parts, machinery, and household appliances.
- Used for making fuse wires.
- Used in nuclear reactors to absorb neutrons.
- Acts as a reducing agent to extract metals like uranium and titanium from their salts.
Tungsten

- Tungsten is a greyish-white, shiny, corrosion-resistant metal with a very high melting point.
- Used to make filaments in incandescent light bulbs.
- Used for electric contacts and arc-welding electrodes.
- Used in steel alloys to increase strength.
Platinum

- Platinum is a shiny, precious, and corrosion-resistant metal.
- Used to make jewellery and watches.
- Used for making electrodes.
- Acts as a catalyst in chemical processes like petroleum refining and hydrogenation.
Recycling of Metals
- Metals come from the earth’s crust and are non-renewable, formed over a long time.
- Metal ores are being used up quickly, so we must use metals wisely and conserve them.
- Recycling means reusing metals that have already been used.
- Metals like tin, copper, aluminium, silver, and gold can be recycled.
Alloys
- An alloy is a uniform mixture of a metal with other metals or non-metals.
- Alloys are designed to have better properties than their components, like high strength, low melting points, or corrosion resistance.
- Alloys are usually harder than their component metals.
- Examples: Brass and bronze (copper alloys), duralumin and magnalium (aluminium alloys), steel and stainless steel (iron alloys), solder and type metal (lead alloys).
Non-Metals
Non-metals are chemical elements which have the capacity to gain electrons and form negative ions called anions.
Sources of Non-metals
Non-metals are found in free state as well as in combined state in the form of compounds.
Properties of Non-metals
Let's study about the properties of non-metals.
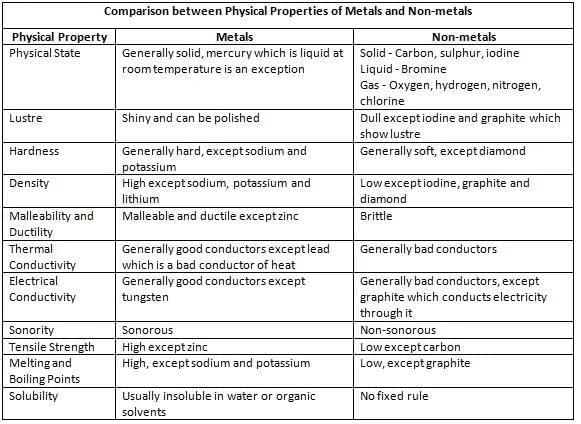
State:
- Non-metals can be solids (like carbon and sulphur), liquids (like bromine), or gases (like oxygen and nitrogen).
- They are usually soft, which means you can easily break or crush them.
- Exception: Diamond (a form of carbon) is a non-metal, but it’s super hard—the hardest natural substance known!
Lustre (Shininess):
- Non-metals usually look dull and don’t shine.
- Exception: Iodine (a non-metal) and graphite (a form of carbon) are shiny and have a nice lustre.
Density:
- Non-metals are generally light because they have low density.
- This means they don’t weigh much for their size.
Malleability:
- Non-metals cannot be hammered into thin sheets—they break if you try.
- This means they are not malleable.
Ductility:
- Non-metals cannot be stretched into thin wires—they snap.
- So, they are not ductile.
Melting and Boiling Points:
- Non-metals usually melt and boil at low temperatures, so they turn into liquids or gases easily.
- Exception: Graphite (a form of carbon) and silicon have high melting points, so they stay solid even at very high temperatures.
Thermal and Electrical Conductivity:
- Non-metals are not good at conducting heat or electricity—they don’t let heat or electric current pass through them easily.
- Exception: Graphite and carbon (when in gaseous form) are good conductors of heat and electricity, which is why graphite is used in things like pencil leads and batteries.
Sonorous (Sound):
- Non-metals don’t make a ringing sound when you hit them—they are not sonorous.
Tensile Strength:
- Non-metals are not very strong and can break easily if you pull or stretch them.
- Exception: Carbon fibre (a form of carbon) is very strong and has high tensile strength, which is why it’s used in things like sports equipment.
Use of Non-metals
Oxygen
- Oxygen is essential for the survival of all living beings.
- Needed for respiration and combustion.
- Liquid oxygen is used as a propellant in spacecraft.
- Used in hospitals to help patients with breathing problems.
- Used in making steel and chemicals like sulphuric acid and nitric acid.
- Used with acetylene for welding and cutting metals.
- Carried in cylinders by firefighters, rescue workers, mountaineers, astronauts, and divers for breathing.
Nitrogen
- Nitrogen is essential for plant and animal growth.
- Used to make ammonia, nitric acid, fertilisers (ammonium salts, urea, potassium nitrate), and explosives (TNT).
- Preserves freshness of packaged food due to its inert nature.
- Liquid nitrogen is used as a cooling agent in refrigerators.
- Helps control the rate of combustion.
- Found in proteins, which are vital for growth.
Hydrogen
- Hydrogen is a colourless, odourless, flammable gas.
- Used to make ammonia (by Haber’s process), hydrochloric acid, and methanol.
- Used to harden vegetable oils (hydrogenation).
- Used with oxygen for cutting and welding metals (oxy-hydrogen flame).
- Used as rocket fuel.
- Considered a future clean fuel.
Sulphur
- Sulphur is a soft, yellow, brittle solid.
- Used to make sulphuric acid, gunpowder, dyes, matches, and fireworks.
- Used in vulcanisation of rubber to improve strength and elasticity.
- Used as an insecticide and fungicide.
- Used in skin ointments and soaps for its fungicidal properties.
Vulcanisation
Vulcanisation is the process of treating rubber with sulphur to make it stronger and more elastic.
Chlorine
- Chlorine is a greenish-yellow toxic gas.
- Used to sterilise drinking water and swimming pool water as a germicide.
- Used to make DDT, BHC, bleaching powder, hydrochloric acid, and PVC.
- Used in paper production, antiseptics, solvents, textiles, insecticides, paints, and petroleum products.
Phosphorus
- Phosphorus is not found in free state; exists in red, yellow, white, and black forms.
- Used to make fertilisers, detergents, and fine chinaware.
- Phosphorus sulphide is used on safety matchbox sides.
- Used in fireworks due to its flammable nature.
- Used in smoke bombs.
- Used as rat poison.
- Used in skin ointments, fumigation, blood purification, and in homeopathic/ayurvedic medicines.
Iodine
- Iodine is a dark grey crystalline solid that sublimes (turns to gas) on heating.
- Tincture of iodine (iodine + alcohol) is used as an antiseptic and disinfectant.
- Silver iodide is used in photography.
- Used in pain balms for pain relief.
- Used in iodised salt to prevent goitre (neck swelling due to iodine deficiency).
Fluorine
- Fluorine is a yellow gas with a strong smell.
- Used to make fluorides and fluorocarbons.
- Used to make teflon for non-stick pans.
- Used in toothpastes (as stannous fluoride) to prevent tooth decay.
- Used in pharmaceutical and agricultural products.
Metalloids
Metalloids are elements that possess properties of both metals and non-metals. Boron, silicon, germanium, arsenic, antimony and tellurium are metalloids.
Properties of Metalloids
- Metalloids appear similar to metals. They are brittle.
- Metalloids such as germanium and silicon act as semiconductors which are important in electronic circuits.
- They form alloys with the metals.
- They are solids under normal conditions.
Metalloids and Their Uses
We will learn about the various uses of some common metalloids later in the chapter.Silicon
- Silicon is not found free in nature; found as sand, the second most abundant element in the earth’s crust.
- Used in silicon chips for computers.
- Used in solar panels to produce electricity from sunlight.
- Used to make glass, transistors, and integrated circuits.
- Used to make silicones for non-stick cookware, fireproof jackets, lubricants, adhesives, and artificial implants.
- Used with ethylene glycol as an antifreeze for airplanes and cars.
Germanium
- Germanium is a shiny, hard, greyish-white metalloid, similar to tin and silicon.
- Used as a semiconductor in transistors and integrated circuits.
- Used in thermal imaging cameras, infrared spectroscopes, and detectors.
- Used as an alloying agent and catalyst in plastic manufacturing.
- Used in rectifiers and as a fluorescent material.
- Used to make specialised glass for military applications.
Arsenic
- Arsenic is a bright, silver-grey, brittle metalloid.
- Used in compounds to preserve wood.
- Used in lead alloys.
- Used in small amounts in the electronics industry.
- Gallium arsenide is used in LEDs.
Antimony
- Antimony is a shiny grey metalloid.
- Used in electronics to make diodes and infrared detectors.
- Used in storage batteries.
- Used to make glass, ceramics, and plastics (as a catalyst).
- Used in musical instruments like organ pipes.
- Used in medicines and paints.
Glossary
- Metals: Elements that lose electrons to form positive ions.
- Mineral: A natural solid found in the earth with a specific chemical makeup and crystal shape.
- Noble Metals: Metals that don’t easily react with non-metals and resist corrosion and oxidation.
- Corrosion: The process where metals get damaged by air, moisture, or chemicals like acids.
- Rusting: The corrosion of iron, forming a reddish-brown layer called rust.
- Non-Metals: Elements that gain electrons to form negative ions.
- Metalloids: Elements that have properties of both metals and non-metals.
Points To Remember
- Elements are divided into two groups based on their properties: metals and non-metals.
- Metals lose electrons to form positive ions.
- Some metals like gold and silver are found in free state, but most exist as compounds like oxides, carbonates, sulphides, and chlorides.
- Metals are usually solid, hard, and shiny at room temperature.
- Metals have high density, except sodium, potassium, and lithium, which are lighter.
- Metals are malleable (can be shaped into sheets) and ductile (can be drawn into wires), except zinc.
- Metals have high melting and boiling points, except sodium and potassium, which melt at lower temperatures.
- Metals are good conductors of heat and electricity, except lead and tungsten.
- Metals are sonorous (make a sound when hit) and have high tensile strength, except zinc.
- Most metals corrode when exposed to damp air, which is a harmful process.
- Corrosion causes a lot of metal to be lost every year.
- Rusting happens when iron reacts with oxygen and water in the air, forming hydrated iron (III) oxide, also called rust (Fe₂O₃·xH₂O).
- For rusting to happen, both air and water must be present.
- Rusting of iron can be stopped by methods like oiling, greasing, painting, galvanisation (coating with zinc), tinning, electroplating, chrome plating, or making alloys.
- Non-metals gain electrons to form negative ions.
|
33 videos|58 docs|7 tests
|
FAQs on Metals and Non-metals Chapter Notes - Chemistry Class 7 ICSE
| 1. What are the main properties of metals like silver, magnesium, tungsten, and platinum? |  |
| 2. Why is recycling of metals important? |  |
| 3. What are alloys and why are they used? |  |
| 4. What are some common non-metals and their uses? |  |
| 5. How do metals differ from non-metals in terms of physical and chemical properties? |  |