NEET PG Exam > NEET PG Notes > Physiology > Chapter Notes: Regulation of Respiration and Applied Physiology
Regulation of Respiration and Applied Physiology Chapter Notes | Physiology - NEET PG PDF Download
Regulation of Respiration
- Ventilatory drive is regulated by neural centers and chemical factors, as summarized in Figure.
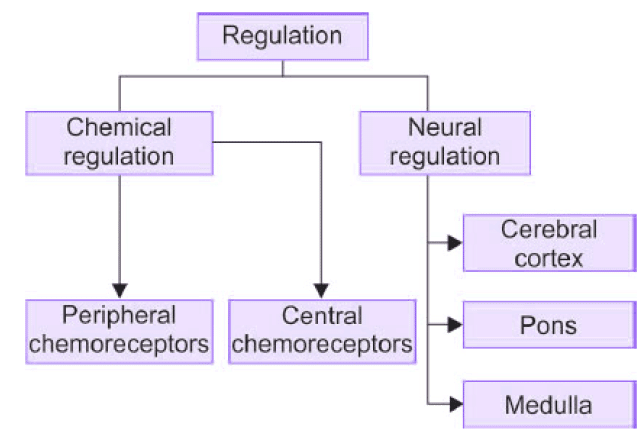
- Chemoreceptors for chemical control include peripheral and central chemoreceptors.
Peripheral Chemoreceptors
- Located in the carotid bodies (near carotid bifurcation, main peripheral chemoreceptor) and aortic bodies (near the arch of the aorta, usually two to three).
- Each carotid and aortic body (glomus) contains type I glomus cells and type II glomus cells.
- Type I glomus cells are associated with cup-like endings of afferent nerves and release neurotransmitters upon excitation.
- Recent studies suggest adenosine triphosphate (ATP) is the key excitatory neurotransmitter during hypoxia, rather than dopamine or acetylcholine as suggested by early studies.
- Type II cells are glia-like, surrounding four to six type I cells, and likely have a sustentacular (supporting) function.
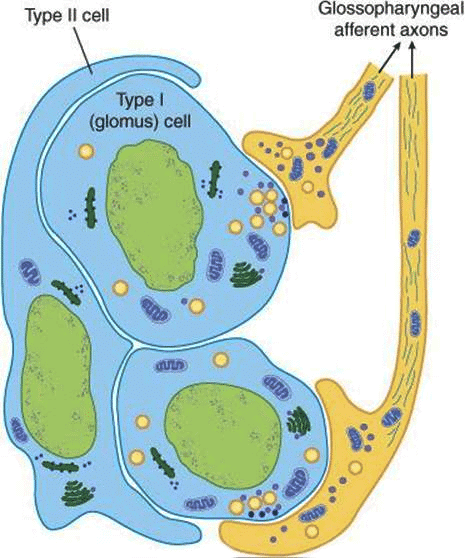 Organization of the carotid body.
Organization of the carotid body. - Respond to increased arterial CO₂ (↑PaCO₂), increased H⁺ (acidosis), decreased O₂ (↓PaO₂), hypoglycemia, hyperinsulinemia, and hypoperfusion.
- Type I glomus cells are sensor cells that respond directly to hypoxia via O₂-sensitive K⁺ channels.
- These chemoreceptors have the highest blood flow in the body (2000 mL/min/100 gm of tissue), meeting O₂ needs with dissolved O₂, making them unresponsive to anemic hypoxia and carbon monoxide (CO) poisoning.
- Mechanism of activation involves ATP as the main neurotransmitter.
- Can be strongly stimulated by cyanide ions but not by anemia or CO poisoning.
- Primary stimulus for peripheral chemoreceptors is hypoxia.
Central Chemoreceptors
- Situated just beneath the ventral surface of the medulla, projecting directly to respiratory centers.
- Respond to increased H⁺ ions in cerebrospinal fluid (CSF) or interstitial fluid (ISF) of the medulla, but not to increased blood H⁺ ions (acidosis) due to the blood-brain barrier (BBB) preventing charged ion passage.
- Blood CO₂ indirectly influences central chemoreceptors by converting to H⁺.
- CO₂ affects both central (70-80% of CO₂ effect) and peripheral (20-30% of CO₂ effect) chemoreceptors.
- Hypoxia does not stimulate central chemoreceptors; instead, it depresses them.
- Primary stimulus for central chemoreceptors is H⁺ ions in CSF or ISF of the medulla.
- Most sensitive stimulus for central chemoreceptors is increased PaCO₂.
Ventilatory Response to PCO₂
- CO₂, not O₂, links metabolism and ventilation.
- There is a linear relationship between respiratory minute volume and alveolar PCO₂.
- In normal subjects inhaling 2%, 4%, and 6% CO₂, respiratory minute volume increases due to increased depth and rate of respiration.
- At >7% CO₂, alveolar and arterial PCO₂ rise abruptly despite hyperventilation, leading to hypercapnia, which depresses the central nervous system, including the respiratory center, causing headache, confusion, and potentially coma (CO₂ narcosis).
Ventilatory Response to PO₂
- The relationship between PO₂ and ventilation is non-linear.
- No increase in ventilation occurs until PaO₂ falls below 60 mm Hg.
- Reasons for lack of response include:
- Hb is a weaker acid than HbO₂, so with less O₂, more Hb inhibits ventilation.
- Increased ventilation washes out CO₂, countering the ventilatory increase.
Ventilatory Response to PCO₂ and PO₂
- The combined effect of CO₂ excess and O₂ lack is more than additive, showing a complex relationship.
- Plotting CO₂ versus ventilation at different fixed O₂ levels produces a fan of curves.
- The slope of the CO₂-ventilation curve increases significantly with decreased O₂ levels.
- The fan of curves intersects at ~PaCO₂ = 37 mm Hg, where lower PaCO₂ causes temporary cessation of respiration (apnea point).
- Since normal PaCO₂ is 40 mm Hg, there is a slight but definite CO₂ drive of the respiratory center.
Ventilatory Response to Composite Effects of PCO₂, pH, and PO₂
- Four curves at different arterial PO₂ levels (40, 50, 60, 100 mm Hg) with increasing PCO₂ at pH 7.4, representing combined effects of PCO₂ and PO₂ on ventilation.
- With hypoxia and hypercapnia, steeper curves indicate a more rapid rise in ventilation, showing hypoxia increases sensitivity to arterial PCO₂.
- Four curves at pH 7.3 represent additive effects of PCO₂ and H⁺ on ventilation, shifted left, indicating the same respiratory stimulation at lower PCO₂ levels in acidosis.
Effect of Baroreceptor Stimulation
- Inhibits respiration but is of minimal physiologic importance.
Effect of Sleep
- Decreased sensitivity to CO₂ during slow-wave sleep, with further decrease during REM sleep.
Voluntary Hyperventilation
- Raises alveolar and arterial PO₂ and lowers PCO₂, causing respiratory depression and potential apnea due to CO₂ washout.
- Breathing pure oxygen raises alveolar PO₂ markedly but does not depress respiration, indicating high PO₂ does not depress respiration.
- Hyperventilation with 5% CO₂ prevents PCO₂ fall, so respiration is not depressed afterward.
- Depression of respiration after hyperventilation is due to CO₂ washout, not PO₂ rise.
Chronic Obstructive Pulmonary Disease (COPD) and Chemoreceptors
- In COPD, CO₂-sensitive chemoreceptors become less sensitive, leading to high arterial CO₂ and low O₂ levels (CO₂ retention).
- The stimulus to breathe in COPD is low arterial O₂ (hypoxia) acting via peripheral chemoreceptors.
Bre breath-Holding
- The breaking point is when breathing can no longer be voluntarily held due to increased CO₂ and decreased O₂.
- Breath-holding can be prolonged by:
- Removal of carotid bodies.
- Breathing 100% O₂ before breath-holding.
- Hyperventilating room air (reduces initial arterial CO₂).
- Encouragement.
Neural Regulation of Respiration
- Involves automatic control by the brainstem (upper medulla and pons) and voluntary control by the cerebral cortex.
- Known as pacemaker cells of respiration or central pattern generator.
- Located on either side of the medulla between the nucleus ambiguus and lateral reticular nucleus.
- Substance P stimulates, and opioids inhibit respiration, limiting opioid use in pain treatment due to respiratory depression.
- Part of the ventral respiratory group (VRG), responsible for respiratory rhythm generation.
DRG and VRG
- Dorsal respiratory group (DRG) and ventral respiratory group (VRG) are in the medulla.
- Lesions of DRG and VRG do not abolish respiratory activity, as they project to pre-Bötzinger pacemaker neurons.
- VRG is subdivided into:
- Bötzinger complex: Contains expiratory neurons, dominant during expiration.
- Pre-Bötzinger complex: Mainly inspiratory neurons, generates respiratory rhythm.
- Rostral VRG: Inspiratory neurons, control airway dilation of larynx and pharynx.
- Caudal VRG: Expiratory neurons, control accessory muscles (abdominal, external intercostal) during forceful expiration.
- DRG contains mainly inspiratory (I) neurons, part of the nucleus tractus solitarius (NTS).
- DRG integrates sensory information from arterial chemoreceptors and lung mechanoreceptors via NTS.
- VRG is a column of neurons firing in phase with respiration, including inspiratory (I) and expiratory (E) neurons.
- All respiratory centers are bilaterally symmetrical.
Pontine Influences
- Modify pacemaker neurons but are not essential for respiration.
Pneumotaxic Center
- Located in the medial parabrachial and Kölliker-Fuse nuclei of the dorsolateral pons.
- Contains both expiratory (E) and inspiratory (I) neurons.
- May fine-tune breathing by smoothing the switch between inspiration and expiration.
- Strong pneumotaxic signal increases breathing rate to 30-40 breaths/min; weak signal reduces it to 3-5 breaths/min.
- Modern view: Not required for eupnea, primarily of historical significance.
Apneustic Center
- Located in the caudal pons, controls depth of breathing by stimulating inspiratory neurons of the pre-Bötzinger complex.
- Tonically active, may integrate afferent information.
- Specific neurons for the apneustic center have not been identified.
- Modern view: Apneustic neurons are not present, term used for historical significance.
Role of Vagal Fibers
- Lung stretching during inspiration initiates impulses in afferent pulmonary vagal fibers, inhibiting inspiratory discharge (I neurons) via the apneustic center and DRG.
- Vagotomy increases depth of inspiration.
- Vagal feedback does not alter the rate of rise of neural activity in respiratory motor neurons.
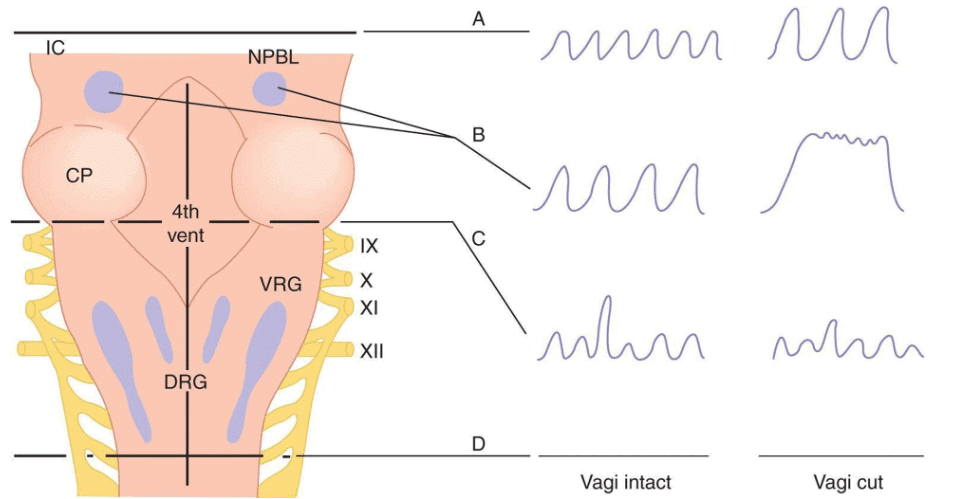
Experimental Basis of Neural Control of Ventilation
Effects of brainstem transections:
- Section A (upper pons, decerebration): Normal rhythmic breathing continues, but voluntary breath-holding is lost. Vagotomy increases depth and slows breathing.
- Section B (mid-pons): Apneustic center intact, pneumotaxic center separated. Apneustic center stimulates inspiratory neurons, increasing breathing depth. With vagi intact: regular, deep, slow breathing. With vagi cut: apneusis (arrest in inspiratory phase) with brief expiration.
- Section C (inferior pons): All pontine tissue separated. With vagi intact or cut: irregular, gasping but rhythmic breathing (pacemaker intact).
- Section D (below medulla): Complete brainstem transection stops respiration (apnea and death).
- Bilateral vagotomy only: Regular, deep, slow breathing (increased tidal volume, slight decrease in respiratory rate).
Hering-Breuer's Reflex
- Self-regulatory negative feedback reflex.
- Receptor: Pulmonary stretch receptors in smooth muscle of large and small airways (bronchi and bronchioles), slowly adapting.
- Stimulus: Lung distension when tidal volume (TV) exceeds 1 L.
- Afferent: Vagus nerve via large myelinated fibers.
- Main effect: Decreases respiratory rate by increasing expiratory time (inflation reflex).
- Activated when TV is more than twice normal (>1 L).
- First description of negative feedback in a physiologic control system (1868).
- Strongest in rabbits, weakest in humans, stronger in infants, activated in adults only when TV >1 L.
Hering-Breuer's Inflation Reflex (Inhibito-Inspiratory Reflex)
- Lung inflation inhibits further inspiratory muscle activity.
- Stretch receptors are stimulated, sending neural signals via vagal afferents to inhibit the apneustic center.
- Facilitates termination of inspiration, prolongs expiration, slows respiratory rate, and may produce apnea.
Hering-Breuer's Deflation Reflex (Excito-Inspiratory Reflex)
- Deflation of lungs inhibits further deflation, initiating inspiratory activity and decreasing expiration duration.
- Initiated by decreased activity in airway stretch receptors or stimulation of proprioceptors activated by lung deflation.
- Conveyed via vagal afferents to brainstem respiratory centers to encourage inspiration.
- In heart-lung transplant patients, absent due to lack of afferent fiber regrowth, but resting breathing pattern remains normal, indicating minimal role in resting respiration.
Head's Paradox Reflex
- Lung inflation augments further inflation, prolonging duration of inspiratory contraction without increasing strength (Henry Head, 1884).
- Receptor: Rapidly adapting pulmonary stretch receptors (RARs), distinct from Hering-Breuer’s slow-adapting receptors (SARs).
- Afferent: Vagus nerve.
- Seen in the “first cry of newborn baby,” absent in adults.
J-Reflex (Pulmonary C-Fiber Receptor)
- Discovered by A.S. Paintal in 1954.
- Location: Juxtapulmonary capillary (J) receptors in alveolar walls or lung interstitial tissue near pulmonary capillaries.
- Afferent: Impulses travel via slowly conducting nonmyelinated fibers (pulmonary C-fiber receptors) in the vagus nerve.
- Stimuli:
- Engorgement of pulmonary capillaries.
- Increased interstitial fluid volume (pulmonary edema, ARDS).
- Pneumonia.
- Pulmonary microembolism.
- Hyperinflation of lungs.
- Chemicals like phenyl diguanide, capsaicin, or volatile agents (e.g., halothane) injected into pulmonary circulation.
- Responses:
- Rapid, shallow breathing.
- Intense stimulation causes apnea.
- Bronchoconstriction.
- Increased mucus secretion.
- Bradycardia and hypotension.
- Muscle weakness.
- J receptors do not control normal breathing, with a postulated role during exercise at high altitude when fluid is trapped in interstitial spaces.
Different Types of Breathing Pattern
Various normal and abnormal breathing patterns:
- Eupnea: Normal breathing rate and pattern.
- Tachypnea: Increased respiratory rate, caused by fever, anxiety, exercise, or shock.
- Bradypnea: Decreased respiratory rate, caused by sleep, drugs, metabolic disorders, head injury, or stroke.
- Hyperpnea: Normal rate but deep respirations, caused by emotional stress or diabetic ketoacidosis.
- Cheyne-Stokes breathing: Gradual increases and decreases in respiration with periods of apnea, caused by severe cardiac failure, brain damage, increased intracranial pressure, or brainstem injury.
- Biot’s breathing: Rapid, deep respirations (gasps) with short pauses, caused by spinal meningitis, CNS causes, or head injury.
- Kussmaul’s breathing: Tachypnea and hyperpnea, caused by diabetic ketoacidosis, uremia, sepsis, salicylates, methanol, aldehyde, or lactic acidosis.
Applied Respiratory Physiology
- Covers high altitude physiology and deep-sea diving physiology.
High Altitude Physiology
- Total barometric pressure decreases at high altitudes (e.g., 5500 m: half sea-level pressure; Mt. Everest summit, 8848 m: 253 mm Hg).
- Oxygen fractional concentration (21%) remains normal, but partial pressure of oxygen (PaO₂) decreases, causing hypoxic hypoxia.
Physiological Adaptations at High Altitude: Acclimatization
- Acclimatization is the process of adjusting to hypoxia to enhance survival and performance.
Changes include
Ventilation:
- Systemic circulation: Hypoxic stimulation of the vasomotor center increases sympathetic activity, causing mild blood pressure increase, moderate heart rate and cardiac output increase. Pulmonary capillary wedge pressure (left arterial pressure) remains low, with no left ventricular dysfunction.
- Pulmonary circulation: Hypoxia causes hypoxic pulmonary vasoconstriction (HPV), increasing pulmonary arterial pressure, leading to pulmonary hypertension. Right atrial pressure does not rise, and right ventricular function remains intact despite extreme hypoxemia.
- Cerebral circulation: Cerebral blood flow (CBF) increases when PaO₂ <60 mm Hg (altitude >2800 m), despite hypocapnia.
- Blood: Hypoxia stimulates erythropoietin release from kidneys, detectable within 2 hours of ascent, promoting red blood cell production (nucleated immature RBCs within days, new RBCs in 4-5 days).
- Oxygen-hemoglobin dissociation curve: Immediate left shift due to respiratory alkalosis, but increased 2,3-diphosphoglycerate (2,3-DPG) shifts it right.
- Tissue changes: Hypoxia induces hypoxia-inducible factor-1α, stimulating vascular endothelial growth factor (VEGF) for angiogenesis, increasing blood flow and oxygen delivery. Mitochondria increase in number, myoglobin facilitates O₂ movement, and cytochrome oxidase content rises.
High Altitude Illnesses
Altitude sickness includes acute mountain sickness (AMS), high-altitude pulmonary edema (HAPE), and high-altitude cerebral edema (HACE), often occurring together.
- Acute Mountain Sickness (AMS):
- Develops 8-24 hours after altitude arrival, lasts 4-8 days.
- Defined as headache with recent altitude gain and symptoms like anorexia, nausea, vomiting, insomnia, dizziness, or fatigue.
- Mild-to-moderate AMS: Characterized by hypoventilation, interstitial edema, and increased sympathetic drive.
- Moderate-to-severe AMS: Associated with white matter edema in the brain due to fluid leak from increased intravascular pressure (cerebral vasodilation).
- High-Altitude Cerebral Edema (HACE):
- Diagnosed by ataxia in AMS or ataxia with mental status changes without AMS.
- Pathophysiology similar to severe AMS.
- High-Altitude Pulmonary Edema (HAPE):
- Occurs within 2-4 days of ascent, commonly on the second night.
- Hypoxic pulmonary vasoconstriction (HPV) is nonhomogeneous, increasing pressure and flow in perfused areas, causing pulmonary hypertension and high-permeability edema with protein and white blood cell leakage.
- Work capacity is reduced in unacclimatized individuals; low-grade, endurance exercise increases work capacity.
- Treatment of AMS:
- Halt ascent and wait for acclimatization.
- Acetazolamide speeds acclimatization.
- Dexamethasone relieves symptoms.
- Analgesics (aspirin, ibuprofen, NSAIDs) for headache.
- Promethazine for nausea and vomiting.
- Oxygen and hyperbaric chambers aid acclimatization.
- Treatment of HAPE/HACE:
- Immediate descent and oxygen administration.
- Drugs (nifedipine, glucocorticoids) are effective; nitric oxide and PDE-5 inhibitors (sildenafil, tadalafil) have unconfirmed results.
Deep-Sea Diving Physiology
- Pressure increases with depth: 33 feet of seawater equals 1 ATM (e.g., 33 feet: 2 ATM; 66 feet: 3 ATM).
- Per Dalton’s law, partial pressures of gases increase with total pressure, affecting biologic effects.
- Per Henry’s law, increased partial pressures increase gas dissolution in body tissues.
- Gases involved: Nitrogen, oxygen, carbon dioxide, each causing significant effects at high pressures.
Oxygen Toxicity at High Pressures
- High tissue PO₂ causes seizures and coma within 30-60 minutes.
- Other symptoms: Nausea, muscle twitching, dizziness, vision disturbances, irritability, disorientation.
Carbon Dioxide Toxicity
- Up to 80 mm Hg PaCO₂ (twice normal alveolar levels), divers tolerate by maximally increasing minute respiratory volume.
- Beyond 80 mm Hg, the respiratory center becomes depressed rather than excited.
Nitrogen Toxicity (Narcosis)
- Similar to alcohol intoxication, called “raptures of the depths.”
- Symptoms:
- 120 feet: Joviality.
- 150-200 feet: Drowsiness.
- 200-250 feet: Clumsiness, impairing work.
- >250 feet (8.5 ATM): Diver becomes almost useless if prolonged.
- Mechanism: Dissolved N₂ in neuronal membrane fatty substances alters ionic conductance, reducing neuronal excitability.
Decompression Sickness (Bends, Compressed Air Sickness, Caisson Disease, Diver’s Paralysis, Dysbarism)
- Caused by sudden decompression after prolonged exposure to high pressure, forming nitrogen bubbles in body fluids.
- Nitrogen bubbles block blood vessels, causing damage.
- Symptoms:
- 85-90%: Pain in joints and muscles (legs, arms), termed “bends.”
- 5-10%: Nervous system symptoms (dizziness, paralysis, collapse, unconsciousness).
- 2%: “Chokes” from microbubbles plugging lung capillaries, causing shortness of breath, severe pulmonary edema, and occasionally death.
The document Regulation of Respiration and Applied Physiology Chapter Notes | Physiology - NEET PG is a part of the NEET PG Course Physiology.
All you need of NEET PG at this link: NEET PG
|
40 docs|9 tests
|
FAQs on Regulation of Respiration and Applied Physiology Chapter Notes - Physiology - NEET PG
| 1. What is the role of the pontine influences in the regulation of respiration? |  |
Ans. The pontine influences, primarily from the pneumotaxic center and the apneustic center located in the pons, play a crucial role in modulating the rhythm and depth of breathing. The pneumotaxic center helps to limit the duration of inhalation, thus promoting a more rhythmic breathing pattern, while the apneustic center stimulates prolonged inhalation. Together, these centers help balance the respiratory cycle and ensure efficient gas exchange.
| 2. What is the Hering-Breuer reflex and how does it affect respiration? |  |
Ans. The Hering-Breuer reflex is a protective reflex that prevents overinflation of the lungs. It is triggered by stretch receptors in the lungs that become activated when the lungs expand during deep inhalation. This reflex sends signals to the respiratory centers in the brain to inhibit further inhalation and initiate exhalation. This mechanism is particularly important during exercise or when taking deep breaths, helping to maintain optimal lung function.
| 3. Can you explain the Head's paradox reflex and its significance in respiration? |  |
Ans. The Head's paradox reflex occurs when the lungs are inflated, but the respiratory centers in the brain are stimulated to breathe due to the activation of lung receptors. This paradoxical response can lead to a feeling of breathlessness even when lung inflation occurs. This reflex is significant because it highlights the complexity of neural regulation of breathing, emphasizing the role of sensory feedback in maintaining respiratory homeostasis.
| 4. What is the J-reflex and what role do pulmonary C-fiber receptors play in this reflex? |  |
Ans. The J-reflex, or the juxtacapillary reflex, is triggered by the activation of pulmonary C-fiber receptors, which respond to pulmonary edema or irritants in the lungs. When stimulated, these receptors send signals to the brain to initiate rapid shallow breathing and may result in a sensation of dyspnea. This reflex serves as a protective mechanism, alerting the body to potential harmful conditions in the lungs.
| 5. How does deep-sea diving physiology impact respiration, and what adaptations are important for divers? |  |
Ans. Deep-sea diving physiology significantly impacts respiration due to increased pressure underwater, which affects gas exchange and oxygen availability. Adaptations such as increased lung capacity, efficient oxygen utilization, and the ability to tolerate high levels of carbon dioxide are crucial for divers. Additionally, divers must be aware of the risks of nitrogen narcosis and decompression sickness, which can arise from changes in pressure during ascent and descent.
Related Searches















