Renal Physiology Chapter Notes | Physiology - NEET PG PDF Download
Renal Physiology
Proximal tubule (PT) is divided into three segments based on ultrastructure:
- S1 segment: First part of proximal convoluted tubule (PCT).
- S2 segment: Second half of PCT and first half of proximal straight tubule (PST).
- S3 segment: Second half of PST.
- S3 segment is located at the corticomedullary junction and is most vulnerable to hypoxic injury.
Two types of cells in collecting duct (CD) and distal convoluted tubule (DCT):
Principal (P) cells:
- Responsible for sodium (Na⁺) reabsorption.
- Responsible for potassium (K⁺) secretion.
- Responsible for water (H₂O) absorption.
Intercalated (I) cells:
- Alpha type: Responsible for acid secretion.
- Beta type: Responsible for bicarbonate (HCO₃⁻) secretion.
Gap junctions (electrical coupling) are present along the lateral cell membrane of PCT but absent in CD.
Acetazolamide inhibits both type IV and type II carbonic anhydrase:
- Primary action on PCT.
- Secondary action on CD.
Functional Anatomy
The nephron is the functional unit of the kidney, with each human kidney containing approximately 1.2 million nephrons.
Nephron components include:
- Renal corpuscle (glomerular capillaries and Bowman’s capsule).
- Proximal tubule.
- Loop of Henle.
- Distal tubule.
- Collecting duct system.
Total length of nephron segments: 45–65 mm.
- Proximal tubule: 15 mm.
- Distal tubules: 5 mm.
- Collecting duct: 20 mm.
Proximal tubule (PT) consists of:
- Proximal convoluted tubule (PCT).
- Proximal straight tubule (PST).
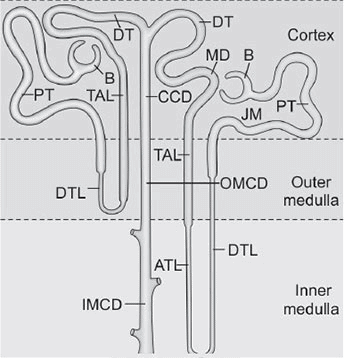
Loop of Henle includes:
- Descending thin limb (DTL).
- Ascending thin limb (ATL).
- Thick ascending limb (TAL).
Connecting tubule: Segment between DCT and CD, unique for producing and releasing renal kallikrein.
Collecting duct (CD) portions:
- Cortical collecting duct (CCD).
- Outer medullary collecting duct (OMCD).
- Inner medullary collecting duct (IMCD).
Collecting duct composed of two cell types:
- Principal (P) cells.
- Intercalated (I) cells.
Difference Between Proximal Tubular Cell And Distal Tubular Cell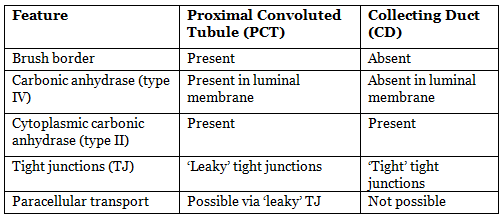
Types of Nephron
- Nephrons are subdivided into cortical and juxtamedullary types.
- Glomerulus of both cortical and juxtamedullary nephrons is located in the cortex.
- Cortical nephron: Maximally extends up to the outer medullary segment.
- Juxtamedullary nephron: Extends deep into the medulla.
Difference Between Two Types of Nephrons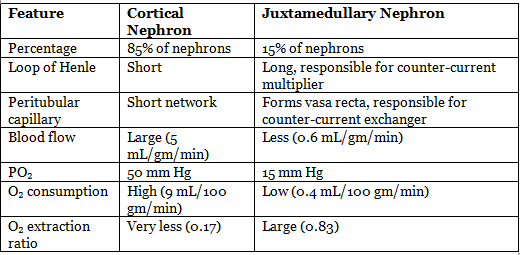
Renal Blood Flow
- Kidney weight: 300 gm.
- Total renal blood flow: 1000–1260 mL/min (20–25% of cardiac output).
- Blood flow by region:
- Cortical: 5 mL/gm/min.
- Outer medulla: 2.5 mL/gm/min.
- Inner medulla: 0.6 mL/gm/min.
- Low blood flow through medulla is necessary to maintain hyperosmolar renal medulla.
- Renal plasma flow (RPF): 700 mL/min.
- Effective renal plasma flow (ERPF): Measured by PAH clearance, 625 mL/min (less than actual plasma flow).
- Oxygen consumption of kidney:
- Total: 18 mL/min.
- Cortex: 9 mL/100 gm/min.
- Inner medulla: 0.4 mL/100 gm/min.
- Artery-venous O₂ difference: 14 mL/L.
Glomerular Filtration Barrier
Composed of three main cellular barriers:
- Fenestrated endothelium.
- Glomerular basement membrane.
- Visceral epithelium cell (podocytes).
Glomerular endothelial cell layer:
- Fenestrated capillary with gaps of 70–90 nm between endothelial cells.
- Coated by glycocalyx containing negatively charged glycosaminoglycan (GAG).
Basement membrane:
- Gel-like acellular meshwork of glycoproteins and proteoglycans.
- Contains negatively charged heparan sulfate.
Visceral epithelial layer (podocytes):
- Podocytes have foot processes that interdigitate to form filtration slits.
- Filtration slits are 25 nm wide, closed by slit diaphragms (4–14 nm gap).
- Slit diaphragms are composed of proteins: nephrin (NPHS1), NEPH-1, podocin (NPHS2), α-actinin 4 (ACTN4), CD2-AP.
Diseases Due To Mutation Of Various Proteins In Glomerular Filtration Membrane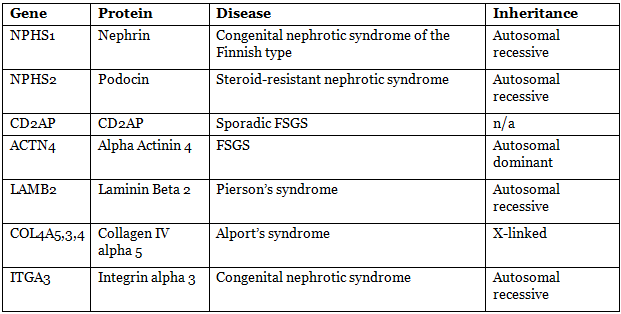
Glomerular Membrane Permeability Depends On
Size of particle:
- Neutral substances <1.8 nm are freely filtered.
- Substances >4.2 nm are not filtered.
- Between 1.8 nm and 4.2 nm, permeability is inversely proportional to diameter.
Charge of the particle:
- Filtration membrane is negatively charged, facilitating filtration of positively charged particles and repelling negatively charged particles.
- Anionic sites (negative charge) are present in all three layers of the glomerular basement membrane (GBM).
Freely filterable substances: Na⁺, K⁺, Cl⁻, HCO₃⁻, urea, creatinine, glucose, amino acids, organic acids (ketone bodies), inulin, PAH, insulin, myoglobin.
Albumin (molecular diameter 3.55 nm):
- Filtration is restricted at the glomerulus.
- Filtered albumin is reabsorbed by megalin/cubilin complex-mediated endocytosis.
- Urinary albumin: 8–10 mg/day.
Glomerular Filtration Rate (GFR)
- GFR: 125 mL/min or 180 L/day.
- Filtration fraction: Ratio of GFR to renal plasma flow (RPF), normally 0.16–0.2.
- Rate of filtration determined by:
- Hydraulic permeability of capillaries.
- Surface area.
- Net filtration pressure (NFP).
- Filtration coefficient (Kf): Product of hydraulic permeability and surface area, Kf = 12.5 mL/min/mm Hg.
- GFR = Kf × NFP.
- NFP is the algebraic sum of:
- Hydrostatic pressures:
- PGC (glomerular capillary): 60 mm Hg.
- PBS (Bowman’s space): 18 mm Hg.
- Oncotic pressures:
- πGC (glomerular capillary): 32 mm Hg.
- πBS (Bowman’s space): 0 mm Hg.
- NFP = [(PGC − PBS) + (πBS − πGC)] = [(60 − 18) + (0 − 32)] = 10 mm Hg.
- Hydrostatic pressures:
Measurement of GFR
Clearance of exogenous substances:
- Substances: Inulin, iohexol, 51Cr EDTA, 125I-iothalamate, 99mTc-DTPA.
- Evaluation: Precise and accurate but costly and time-consuming.
Clearance of endogenous blood substances:
- Serum creatinine: Insufficiently sensitive for detecting chronic renal disease (CRD).
- Creatinine clearance: Not recommended due to errors in urine collection, except in patients with highly abnormal muscle mass or vegetarian diet.
- Serum cystatin C: More sensitive than serum creatinine for detecting GFR reduction in the range 70–40; better than creatinine in children.
Regulation of GFR
Regulated by:
- Blood pressure (autoregulation).
- Resistance of afferent and efferent arterioles.
Autoregulation: Maintains constant renal blood flow or GFR over a renal perfusion pressure range of 80–170 mm Hg.
Mechanisms of autoregulation:
- Myogenic mechanism: Contraction or relaxation of arteriolar smooth muscle in response to changes in vascular pressure, mediated by Ca²⁺.
- Tubuloglomerular feedback (TGF) mechanism: Mediated by macula densa cells.
Effects of Afferent and Efferent Arteriole Resistance Change on GFR and RPF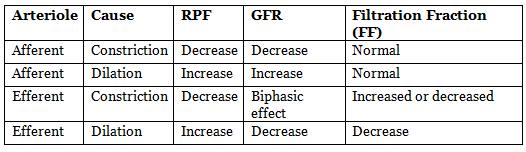
- Afferent arteriole constriction reduces GFR.
- Efferent arteriole constriction effect on GFR depends on severity:
- Modest constriction increases GFR.
- Severe constriction (more than threefold increase in resistance) reduces GFR.
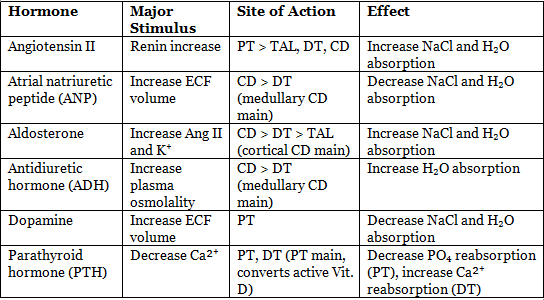
Effect of Angiotensin II on Kidney
- Direct effects:
- Low concentrations stimulate Na⁺/H⁺ exchange in proximal tubule, increasing Na⁺, Cl⁻, and bicarbonate reabsorption.
- Release of aldosterone from adrenal cortex:
- Aldosterone acts on distal and collecting tubules to retain Na⁺ and excrete K⁺ and H⁺.
- Altered renal hemodynamics:
- Reduces renal blood flow by constricting renal vascular smooth muscle (efferent > afferent).
Effect on GFR:
- Constriction of afferent arterioles reduces GFR.
- Contraction of mesangial cells reduces GFR.
- Constriction of efferent arterioles increases GFR.
- Outcome depends on physiological state:
- Normally, GFR is slightly reduced.
- During renal artery hypotension, efferent arteriole effects predominate, increasing GFR.
- Blockade of renin-angiotensin system may cause acute renal failure in patients with bilateral renal artery stenosis or unilateral stenosis with a single kidney.
Juxtaglomerular Apparatus
Components:
- Juxtaglomerular (JG) cells:
- Modified smooth muscle cells in the tunica media of the afferent arteriole.
- Contain renin-containing granules (granular cells).
- Renin is an aspartyl protease (enzyme).
- Macula densa:
- Specialized tubular epithelium of the thick ascending limb of the loop of Henle.
- Marks the beginning of DCT.
- Chemoreceptor cells that regulate tubuloglomerular feedback mechanism.
- Lacis cells (extraglomerular mesangial cells or Goormaghtigh/Polkissen cells).
Mesangial Cells
Located in two sites:
Glomerular mesangial cells:
- Located between glomerular capillary loops.
- Contractile cells that regulate glomerular filtration.
- Relaxation (caused by PGE2, ANP, cAMP, dopamine) increases GFR.
Extraglomerular mesangial cells (Lacis cells):
- Part of the juxtaglomerular apparatus.
- Secrete glomerular basement membrane.
- Act as anti-inflammatory cells.
Stimuli for increased renin release:
- Renal hypoperfusion and hypotension (most potent).
- Sympathetic stimulation.
- Decreased NaCl in macula densa.
- Renal ischemia.
Three pathways control renin secretion from JG cells:
Macula densa pathway:
- Increased NaCl reabsorption by macula densa cells releases adenosine, inhibiting renin release.
- Decreased NaCl flux releases prostaglandin, stimulating renin release.
- Adenosine acts via A1 adenosine receptor to inhibit renin release.
Intrarenal baroreceptor pathway:
- Baroreceptors in JG cells sense afferent arteriolar pressure.
- Lowering of pressure (e.g., renal artery stenosis) leads to degranulation of JG cells, increasing renin secretion.
Beta-adrenergic receptor pathway:
- Mediated by norepinephrine release from postganglionic sympathetic nerves.
- Activation of beta1 receptors on JG cells enhances renin secretion.
Tubuloglomerular Feedback (TGF)
- Feedback mechanism linking NaCl concentration at macula densa with control of renal arteriolar resistance.
- Decreased NaCl at macula densa causes:
- Dilation of afferent arterioles.
- Increased renin release.
- Sensor: Macula densa senses Na⁺ and Cl⁻ via Na-K-2Cl cotransporter in apical membranes.
- Increased Na⁺ and Cl⁻:
- Increases Na-K-ATPase activity and ATP hydrolysis, producing more adenosine.
- Adenosine acts via A1 receptors on macula densa cells to increase Ca²⁺ release to vascular smooth muscle in afferent arterioles, causing vasoconstriction and decreased GFR.
- Decreased NaCl triggers the opposite mechanism.
- Cl⁻ concentration is a more critical modulator than Na⁺ in the macula densa pathway.
Glomerulotubular Balance
- Increased GFR increases solute and water reabsorption, primarily in the proximal tubule, maintaining a constant percentage of solute reabsorption.
- Cause: Increased oncotic pressure in peritubular capillaries due to high GFR, enhancing Na⁺ reabsorption from the tubule.
Clearance
- Clearance: Volume of plasma per unit time from which a specific substance is completely removed (mL/min).
- Formula: C = (U × V) / P
- C = clearance.
- U = urine concentration of the substance.
- V = urine flow rate.
- P = plasma concentration of the substance.
- Clearance represents: (Filtration + Secretion − Absorption) / Plasma concentration.
- For substances neither absorbed nor secreted (e.g., inulin), clearance = GFR (125 mL/min).
- Example (inulin clearance):
- Plasma inulin concentration: 4 mg/L.
- Urine volume: 0.1 L/h, urinary inulin concentration: 300 mg/L.
- Inulin excretion: 0.1 L/h × 300 mg/L = 30 mg/h.
- Clearance: (30 mg/h) / (4 mg/L) = 7.5 L/h.
- PAH clearance:
- Gives effective renal plasma flow (ERPF): 625 mL/min.
- PAH is secreted by active transport.
- Filtered load is linear with plasma level, but secretion reaches a maximum (TmPAH).
- At high PAH concentrations, clearance decreases, approaching inulin clearance, giving falsely lower RPF.
- Clearance hierarchy: CPAH >> CK⁺ >> CCr > CIn >> CUrea >> CNa⁺ >> CGlucose.
Tubular Reabsorption and Secretion
- Tonicity of tubular fluid:
- End of PCT: Isotonic (solute reabsorption proportional to water).
- Descending limb of loop of Henle: Hypertonic (impermeable to solutes, only water reabsorption).
- Ascending limb: Initially isotonic, then hypotonic (impermeable to water, only solute reabsorption; known as diluting segment).
- Calculations:
- Filtered load = GFR × Plasma concentration.
- Reabsorption rate = Filtered load − Excretion rate.
- Secretion rate = Excretion rate − Filtered load.
- Example (glucose excretion):
- Plasma glucose: 400 mg/dL (4 mg/mL).
- GFR: 125 mL/min.
- Filtered load: 125 × 4 = 500 mg/min.
- Tm (glucose): 375 mg/min.
- Excretion: 500 − 375 = 125 mg/min.
Sodium Reabsorption
- Occurs in all tubule segments except the thin descending limb.
- Active reabsorption except in thin ascending limb (passive).
- Mechanisms by segment:
- PCT (65%):
- Major: Na⁺ entry coupled to H⁺ secretion via NHE3 antiporter.
- Additional Na⁺ enters in symport with glucose (SGLT), amino acids, phosphate, citrate, and lactate.
- All Na⁺ transported to interstitium via basolateral Na,K-ATPase.
- PST: Cl⁻-driven Na⁺ transport (paracellular pathway).
- DTL: No reabsorption, permeable only to H₂O.
- ATL: Passive (paracellular pathway).
- TAL (25%):
- Major transporter: Na-K-2Cl symporter (NKCC).
- LoopPolicy: Loop diuretics (e.g., furosemide) inhibit NKCC.
- Contains K⁺ channels (ROMK) that recycle K⁺ to lumen and interstitium.
- Some Na⁺ moves paracellularly due to lumen-positive potential.
- Mutations in NKCC cause Bartter’s syndrome.
- DCT (6%):
- Apical Na-Cl symporter (NCC), inhibited by thiazide diuretics.
- Mutation of NCC causes Gitelman’s syndrome.
- CD (~3%):
- Na⁺ reabsorption via apical Na⁺ channels (ENaC) in principal cells.
- Gain-of-function mutations of ENaC cause Liddle syndrome.
- Aldosterone increases open ENaC and Na,K-ATPase on basolateral membrane.
- ENaC blocked by triamterene or amiloride diuretics.
- Atrial natriuretic peptide (ANP) inhibits ENaC, causing natriuresis and diuresis.
- PCT (65%):
Atrial Natriuretic Peptide (ANP) and Brain Natriuretic Peptide (BNP)
- Secretion:
- ANP: Cardiac atria.
- BNP: Cardiac ventricles.
- Stimulus: Rise in blood pressure and increased extracellular fluid (ECF) volume.
- Actions:
- Reduce blood pressure by decreasing total peripheral resistance.
- Enhance urinary excretion of NaCl and water.
- Increase capillary permeability.
- Dilate afferent arterioles, constrict efferent arterioles, and relax mesangial cells, causing modest GFR increase with little change in renal blood flow.
- Inhibit NaCl reabsorption by medullary collecting duct (MCD).
- Inhibit ADH-stimulated water reabsorption across CD.
- Reduce ADH secretion from posterior pituitary.
- ANP causes more profound natriuresis and diuresis than BNP.
- ANP in brain (hypothalamus) opposes angiotensin II effects, lowering blood pressure and promoting natriuresis.
Water Reabsorption
- Passive, following osmotic gradient.
- Total glomerular filtered load: ~180 L/day.
- Urine output varies: 500 mL (1400 mOsm/L) to 23.3 L (30 mOsm/L).
- Facilitated by aquaporins (water channels).
- Seven aquaporins expressed in kidneys:
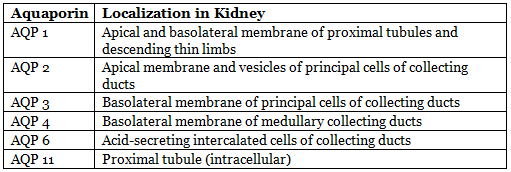
- Vasopressin (ADH) acts on AQP-2 via V2 receptor, increasing cAMP, causing rapid insertion of AQP-2 vesicles into apical membrane of principal cells.
Concentration and Dilution of Urine
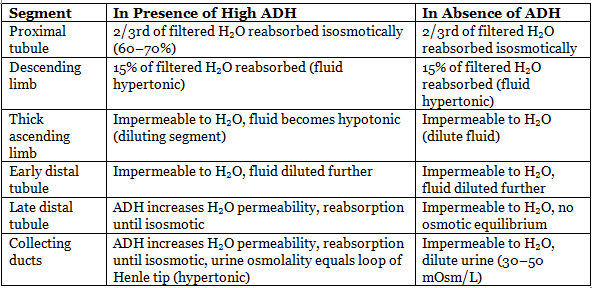 Maximum H₂O reabsorption: Proximal tubule (60–70%), descending limb (15%).
Maximum H₂O reabsorption: Proximal tubule (60–70%), descending limb (15%).
With ADH:
- Cortical CD: 10% H₂O reabsorbed.
- Medullary CD: 4.7% H₂O reabsorbed.
- Maximum hypertonic filtrate: Tip of loop of Henle or CD end.
- Maximum hypotonic filtrate: DCT.
Without ADH:
- CD reabsorbs only 2% H₂O.
- Maximum hypertonic filtrate: Tip of loop of Henle or CD end.
- Maximum hypotonic filtrate: Connecting tubule (CT) end.
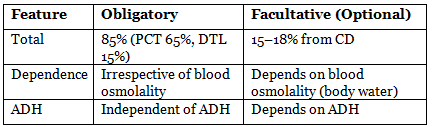
Obligatory and Facultative Water Absorption
Free Water Clearance
Measures solute-free water saved or eliminated in urine (CH2O).
Formula: CH2O = V − COSM
- V = urine flow rate.
- COSM (osmolar clearance) = (UOSM × V) / POSM.
- Simplified: CH2O = V (1 − UOSM / POSM).
Normal human (70 kg):
- Excretes ~600 mOsmol solute/day.
- Plasma osmolality: 300 mOsm/L.
- Isosmotic urine requires 2.0 L H₂O/day, CH2O = 0.
Conditions:
- UOSM / POSM > 1 (concentrated urine): CH2O negative (water deprivation, SIADH).
- UOSM / POSM = 1 (isosmotic urine): CH2O = 0 (loop diuretic treatment).
- UOSM / POSM < 1 (dilute urine): CH2O positive (high water intake, central or nephrogenic diabetes insipidus).
Glucose Reabsorption
Secondary active transport in proximal tubules:
- SGLT2 (PCT, S1, S2 segments): 90% of glucose absorption.
- SGLT1 (PST, S3 segment): 10% of glucose absorption.
Transport maximum (TmG):
- Males: 375 mg/min.
- Females: 300 mg/min.
Renal threshold:
- Arterial: 200 mg/dL.
- Venous: 180 mg/dL.
Splay effect: Deviation in renal threshold due to nephron heterogeneity and variable nephron activity.
SGLT2 inhibitors (e.g., dapagliflozin, canagliflozin): Used in type II diabetes mellitus.
SGLT2 mutation: Causes familial renal glycosuria.
Transport Maximum (Tm)
- Limit to the rate of active reabsorption or secretion due to saturation of transport systems.
- Occurs when tubular load exceeds carrier protein and enzyme capacity.
- Tm for actively reabsorbed substances:
- Glucose: 375 mg/min.
- Lactate: 75 mg/min.
- Plasma proteins: 30 mg/min.
- Urate: 15 mg/min.
- Amino acids: 1.5 mM/min.
- Tm for actively secreted substances:
- PAH: 80 mg/min.
- Creatinine: 16 mg/min.
- No Tm for Na⁺ (actively reabsorbed).
Potassium: Reabsorption and Secretion
- Only electrolyte both reabsorbed and secreted.
- Reabsorption:
- PCT: 65%.
- Loop of Henle: 25%.
- <10% reaches distal nephron.
- Active reabsorption in PCT.
- Secretion in DCT (maximum secretion):
- Decreased when Na⁺ reaching DCT is low.
- Depends on dietary K⁺ load (20–180%).
- 20–40% of secreted K⁺ reabsorbed in CD.
- K⁺ secretion decreases when H⁺ secretion increases.
- In DCT, Na⁺ reabsorption competes with K⁺ and H⁺ secretion.
- Clinical correlations:
- Hypokalemic alkalosis.
- Hyperkalemic acidosis.
Acid Secretion
- Occurs in PCT, DCT, and CD.
- Mechanisms in PCT:
- Na⁺-H⁺ exchanger (secondary active transport): Primary mechanism for acid secretion.
- For each H⁺ secreted, 1 Na⁺ and 3 HCO₃⁻ reabsorbed indirectly via basolateral Na-HCO₃ symporters and Cl-HCO₃ antiporters.
- Bicarbonate efflux: Passive in symporters, active in Na⁺ symport.
- Secreted H⁺ does not acidify urine; aids Na⁺ and HCO₃⁻ reabsorption.
- High-capacity, low-gradient system (high capacity, no acidification).
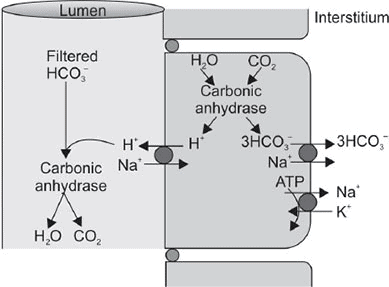 Acid secretion in PCT.
Acid secretion in PCT.
- Mechanisms in DCT/CD:
- ATP-driven H⁺ pump (H-ATPase) and H⁺-K⁺ ATPase (H⁺-K⁺ ATPase less active).
- Secreted H⁺ acidifies urine.
- Low-capacity, high-gradient system (low capacity, significant acidification).
- Factors affecting acid secretion:
- Increased intracellular PCO₂ increases acid secretion.
- K⁺ depletion increases acid secretion.
- Carbonic anhydrase inhibition decreases acid secretion.
- Aldosterone increases Na⁺ reabsorption, K⁺, and H⁺ secretion.
- Clinical correlations:
- Hypokalemia causes alkalosis.
- Hyperkalemia causes acidosis.
- Net acid excretion = Excreted titratable acids + Excreted NH₄⁺ − Excreted HCO₃⁻.
- Titratable acid:
- Measured by titrating urine with NaOH to blood pH (7.40).
- Represents H⁺ combined with urinary buffers (e.g., phosphate, creatinine).
- Phosphate (H₂PO₄⁻) is the largest component.
Urinary Buffers
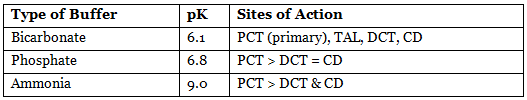
- Limiting urine pH: 4.5 (1000 times H⁺ concentration).
- Bicarbonate: Most important in PCT.
- Ammonia: Most important in distal tubules.
- Phosphate: More important than ammonia in distal tubules due to high phosphate concentration and low ammonia activity.
Hormonal Regulation of Tubular Reabsorption
Counter Current Mechanism
Process making renal medullary interstitial fluid hyperosmotic.
Two parts:
- Counter current multiplier.
- Counter current exchanger (vasa recta).
Counter current multiplier:
- Major contributors:
- TAL (thick): Active transport of Na⁺, K⁺, Cl⁻.
- CD: Active transport of Na⁺.
- MCD: Facilitated diffusion of urea (40–50%).
- ATL (thin): Diffusion of NaCl (minor role).
- Steps:
- Loop of Henle fluid starts at 300 mOsm/L.
- TAL actively absorbs NaCl, creating a 200 mOsm/L gradient (tubular fluid: 200 mOsm/L, interstitial: 400 mOsm/L).
- DTL water absorption equilibrates to 400 mOsm/L.
- Proximal tubule fluid (300 mOsm/L) pushes hyperosmotic fluid to ascending limb.
- TAL pumps more ions, raising interstitial osmolarity to 500 mOsm/L.
- DTL equilibrates with interstitial fluid.
- Process repeats, trapping solutes in medulla, raising interstitial osmolarity to 1200–1400 mOsm/L.
Medullary interstitial fluid osmolality (maximally concentrated urine):
- ~1200 mOsm/kg H₂O at papilla.
- 50% (~600 mOsm/kg): Na⁺ (300 mOsm/kg) and Cl⁻ (300 mOsm/kg).
- 40–50% (500–600 mOsm/kg): Urea.
Urea is the single most important substance for medullary tonicity.
Countercurrent Exchange in the Vasa Recta
- Descending limb of vasa recta:
- Becomes hyperosmotic due to water diffusion out and solute diffusion in from interstitial fluid.
- Ascending limb:
- Solutes diffuse back to interstitial fluid.
- Water diffuses back into vasa recta.
- Despite large fluid and solute exchange, U-shaped vasa recta prevent net dilution of interstitial fluid concentration.
- Vasa recta maintain, but do not create, medullary hyperosmolarity.
Important Points of Countercurrent Mechanism
- Longer loop of Henle increases medullary interstitial osmotic gradient, determining concentration ability.
- Counter current multiplier establishes the gradient; vasa recta exchange maintains it.
- Multiplier is active; exchanger is passive.
- With ADH, water moves from collecting duct.
- Cortical CD concentrates urine to isotonicity; medullary CD concentrates to the maximum interstitial gradient.
Endocrine Functions of Kidney
Produces three hormones:
- 1,25-dihydroxycholecalciferol.
- Renin.
- Erythropoietin (EPO).
Erythropoietin (EPO):
- Source:
- Adults: 85% kidneys, 15% liver.
- Also in spleen, salivary glands, brain, uterus.
- Kidney: Produced by interstitial cells in peritubular capillary bed.
- Liver: Perivenous hepatocytes.
- Stimulus: Hypoxia (main), cobalt salts, androgens, catecholamines (via α-adrenergic mechanism), alkalosis (high altitude).
- Function: Increases committed stem cells in bone marrow, enhances RBC production, mainly affects colony-forming unit-erythroid (CFU-E) cells.
- Metabolism: Inactivated in liver, half-life ~5 hours.
|
40 docs|9 tests
|
FAQs on Renal Physiology Chapter Notes - Physiology - NEET PG
| 1. What are the different types of nephrons, and how do they differ in function? |  |
| 2. What is the glomerular filtration barrier, and what role does it play in kidney function? |  |
| 3. How is glomerular filtration rate (GFR) defined, and what factors influence it? |  |
| 4. What is the juxtaglomerular apparatus, and what is its significance in renal physiology? |  |
| 5. What is tubuloglomerular feedback (TGF) and how does it help in regulating GFR? |  |
















