Chemical Reaction: Nucleophilic Substitution Reactions | Chemistry for JEE Main & Advanced PDF Download
The reactions of haloalkanes may be divided into the following categories:
1. Nucleophilic substitution
2. Elimination reactions
3. Reaction with metals.
Nucleophilic substitution reaction
Chemical reactions of alkyl halide:
Those organic compounds in which an sp3 hybridized carbon is bonded to an electronegative atom or group can undergo two types of reaction e.g. substitution reactions in which the electronegative atom or group is replaced by another atom or group. The second is the elimination reaction in which the electronegative atom or group is eliminated along with hydrogen from an adjacent carbon. The electronegative atom or group which is substituted or eliminated is known as leaving group. 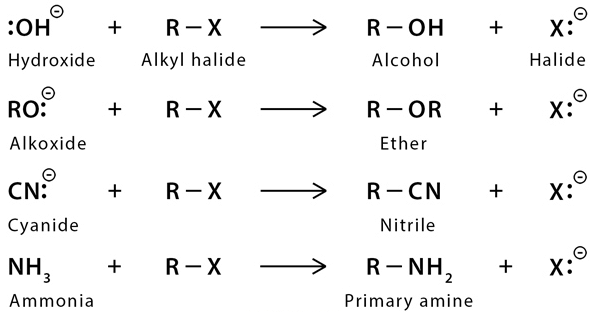 Nucleophilic Substitution Reaction
Nucleophilic Substitution Reaction
Because of more electronegativity of the halogen atom, it has a partial negative charge and partial positive cha develops on the carbon atom.
X = F, Cl, Br, I
Due to this polar carbon - halogen bond alkyl halides shows nucleophilic substitution and elimination reaction.
There are two important mechanisms for the substitution reaction:
- A nucleophile is attracted to the partially positively charged carbon. As the nucleophile approaches the carbon, it causes the carbon - halogen bond to break heterolytically (the halogen keeps both of the bonding electrons.)
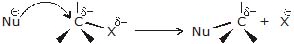
- The carbon-halogen bond breaks heterolytically without any assistance from the nucleophile, with the help of polar protic solvent and carbocation is formed (solvolysis). Formed carbocation then reacts with the nucleophile to form the substitution product.
Bimolecular nucleophilic substitution reaction (SN2)
The mechanism of SN2 reaction
transition state
Characteristic of SN2
It is bimolecular, unistep process
- It is the second-order reaction because in the Rds two species are involved
- Kinetics of the reaction → rate ∝ [alkyl halide] [nucleophile]
rate = k[alkyl halide] [nucleophile]
If the concentration of alkyl halide in the reaction mixture is doubled, the rate of the nucleophilic substitution reaction is double. If the concentration of nucleophile is doubled the rate of reaction is also double. If the concentration of both is doubled then the rate of the reaction quadruples. - Energetics of the reaction:
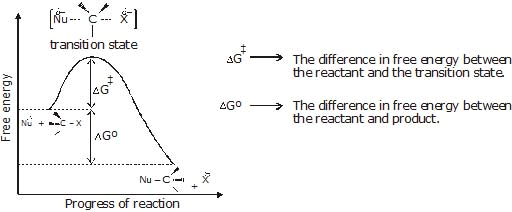
Figure: A free energy diagrams for a hypothetical SN2 reaction that takes place with a negative DGº - No intermediates are formed in the SN2 reaction, the reaction proceeds through the formation of an unstable arrangement of atoms or group called transition state.
- Stereochemistry of SN2 reaction: As we saw earlier, in an SN2 mechanism the nucleophile attacks from the backside, that is from the side directly opposite to the leaving group. this mode of attack causes an inversion of configuration at the carbon atom that is the target of nucleophilic attack. This inversion is also known as Walden inversion.
Factors affecting the rate of an SN2 reaction
A number of factors affect the relative rate of SN2 reaction, the most important factors are:
- Structure of the substrate
- Concentration and reactivity of the nucleophile
- Effect of the solvent
- Nature of the leaving group
1. Effect of the structure of the substrate:
Order of reactivity in SN2 reaction : - CH3 > 1º > 2º >> 3º (unreactive)
the important factor behind this order of reactivity is a steric effect. Very large and bulky groups can often hinder the formation of the required transition state and crowding raises the energy of the transition state and slows down the reaction.
Relative rates of reactions of an alkyl halide in SN2 reaction
Substituent | Compound | Relative rate |
Methyl | CH3X | 30 |
1° | CH3CH2X | 1 |
2° | (CH3)2CHX | 0.02 |
Neopentyl | (CH3)3CCH2X | 0.00001 |
3° | (CH3)3CX | ~0 |
2. Concentration and reactivity of the nucleophile
According to the kinetics of SN2 increasing the concentration of the nucleophile increases the rate of an SN2 reaction. The nature of nucleophile strongly affects the rate of an SN2 reaction. A stronger nucleophile is much more effective than a weaker one. For example, we know that a negatively charged nucleophile is more reactive than its conjugate acid e.g. HO- > H2O, RO- > ROH.
Steric effects on nucleophilicity
3. The effect of the solvent: In polar protic solvent large nucleophiles are good, and the halide ions show the following order: I- > Br- > Cl- > F- (in polar protic solvent)
This effect is related to the strength of the interaction between nucleophile and solvent molecules of polar protic solvent forms a hydrogen bond to nucleophiles in the following manner. Because small nucleophile is solvated more by the polar protic solvent thus its nucleophilicity decreases and the rate of SN2 decreases
Relative nucleophilicity in a polar protic solvent:
SH+ > CN- > I- > OH- > N3- > Br- > ACO- > Cl- > F- > H2O
So, polar protic solvents are not useful for the rate of SN2, if the nucleophile is anionic. But polar aprotic solvent does not have any active hydrogen atom so they can not forms an H bond with nucleophiles. Polar aprotic solvent has a crowded positive centre, so they do not solvate the anion appreciably therefore the rate of SN2 reactions increased when they are carried out in a polar aprotic solvent.
Examples of a polar aprotic solvent.
In DMSO, the relative order of reactivity of halide ions is: F- > Cl- > Br- > I-
4. The nature of the leaving group: The best-leaving groups are those that become the most stable ion after they leave because leaving group generally leave as a negative ion, so those leaving groups are good, which stabilise negative charge most effectively and weak base do this best, so weaker bases are good leaving groups. A good leaving group always stabilize the transition state and lowers its free energy of activation and thereby increases the rate of the reaction.
Order of leaving ability of halide ion: I- > Br- > Cl- > F-
Other leaving groups are
Strongly basic ions rarely act as leaving group:
Examples of SN2 reactions of alkyl halide →
| Nucleophile | Product | Class of Product | |
| R - | Alkyl halide | ||
| R - | Alcohol | ||
| R - | Ether | ||
| R - | Thiol(mercaptan) | ||
| R - | Thioether (sulphide) | ||
| R - | Amine | ||
| R - | Azide | ||
| R - | Alkyne | ||
| R - | Nitrile | ||
| R - COO - R | Ester | ||
| [R - PPh3]+ | Posphonium salt |
Question 1: Complete the following reactions with mechanism
(a)
Sol.
(b) ?
Sol. (p-Nitroanisole)
(c) Ph - CH2Cl
Sol. CH3-CH2-O! is present in excess and it is stronger nucleophile than Ph - O! so the product is Ph-CH2 - OEt
(d) CH3 - C ≡ CH X
Y
Sol.
(e) Ph3 → Salt
Sol.
Question 2: When the concentration of alkyl halide is tripled and the concentration of OH- ion is reduced to half, the rate of SN2 reaction increases by :
(A) 3 times (B) 2 times (C) 1.5 times (D) 6 times
Ans: c
Unimolecular Nucleophilic Substitution Reaction (SN1) 
Characteristics of SN1 reactions
- It is a unimolecular, two-step process and intermediate is formed, (intermediate is carbocation)
- It is a first-order reaction
- Kinetics of the reaction, Rate ∝ [Alkyl halide]
Rate = k[(CH3)3C-X]
The rate of SN1 reaction is independent of the concentration and reactivity of nucleophile. - Stereochemistry of SN1 reaction:
In the SN1 mechanism, the carbocation intermediate is sp2 hybridized and planar. A nucleophile can attack the carbocation from either face if the reactant is chiral than after the attack of nucleophile from both faces gives both enantiomers of the product, which is called recemization.Mechanism of recemization (SN1):
- Energetics of the SN1

Figure: free energy diagram for the SN1 reaction.
Factors affecting the rates of SN1
The structure of the substrate:
The Rds of the SN1 reaction is an ionization step, in this step form a carbocation. This ionisation is a strongly endothermic process, rate of SN1 reaction depends strongly on carbocation stability because carbocation is the intermediate of SN1 reaction which determines the energy of activation of the reaction.
SN1 reactivity : 3º > 2º > 1º > CH3 - X- Concentration and reactivity of the nucleophile:
The rate of SN1 reactions are unaffected by the concentration and nature of the nucleophile - Effect of the solvent:
Because to solvate cations and anions so effectively the use of polar protic solvent will greatly increase the rate of ionization of an alkyl halide in any SN1 reaction. It does this because solvation stabilizes the transition state leading to the intermediate carbocation and halide ion more than it does the reactant, thus the energy of activation is lower.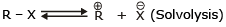
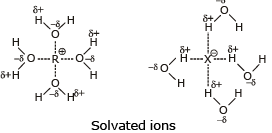
- The nature of the leaving group:
In the SN1 reaction, the leaving group begins to acquire a negative charge as the transition state is reached the stabilisation of this developing negative charge at the leaving group stabilizes the transition state and: this lowers the free energy of activation and thereby increases the rate of reaction.
Comparison of SN1 and SN2 reactions :
|
| SN1 | SN2 |
(i) | Effect of the nucleophile | Nucleophile strength is not important | A stronger nucleophile is required |
(ii) | Effect of the substrate | 3° > 2° > 1° > CH3X | CH3X > 1° >2° |
(iii) | Effect of solvent | Good ionizing solvent required | It goes faster in a less polar solvent if Nud is present |
(iv) | Kinetics | Rate = k [R-X] | Rate = k[R-X] [Nu°] |
(v) | Stereochemistry | Racemisation | Walden inversion |
(vi) | Rearrangement | common | Impossible |
Question 3: Predict the compound in each pair that will undergo solvolysis (in aqueous ethanol) more rapidly.
Sol. (a) II > I (b) II > I (c) I > II (d) II > I (e) II > I
Question 4: Give the solvolysis products expected when each compound is heated in ethanol
(a)
(b)
(c)
(d)
Sol. (a)
(b)
(c)
(d)
Question 5: The rate of SN1 reaction is fastest with
Ans. (A)
The reaction of RX with aq. KOH
-
R - OH + KCl
- CH3-CH2-Cl
CH3-CH2-OH
- CH3-CH
CH3-CH
CH3-CHO
- CH3-C
CH3-C
CH3COOH
- C - C - C - C - Br
C - C - C - C - OH
- 14C = C - C - I
14C = C - C - OH + C = C - C14 - OH
Other Nucleophilic reaction of R - X :-
WillionSon's Ether Synthesis: (SN2)
- EtONa + Me - Cl
EtOMe
EtOMe
Rate (2) > (3) 2 is better method. (Due to less steric hindrence)- MeONa + PhCl
No reaction
+ NaCl
- Me3CO!NaÅ + MeCl
Me3COMe + NaCl
- MeO!NaÅ + Me3C - Cl
+ MeOH + NaCl
- PhONa + Me3C-Cl
+ PhOH + NaCl
- Me3CONa + Ph-Cl
No. reaction
Me3CO-Ph can not be prepared by Williamson's ether synthesis. -
-
+ HCl
+ HBr
-
(Pyridinium salt)
Hydrolysis of Ether
- MeOEt
Et -
+ Me - OH
Reaction of ether with HI :
- Me - O - Et
(Due to formation of more stable carbocation)
With moist and dry Ag2O :
- 2Me - Cl
Me - O - Me + 2AgCl
- Me - Cl + EtCl
Me - O - Et + Me - O - Me + EtOEt
SN1 (Nucleophilic substitution intramolecular )
Darzon's process
Mechanism:
R - Cl + O = S = O (g)
Note : (1) In SNi retention of the configuration takes place.
Note : (2) In presence of pyridine above reaction follow the SN2 reaction mechanism.
SNNGP(Neighbouring group participation)
An increase in the rate of SN reaction due to attack of internal nucleophile is called SNNGP is also known as Anchimeric assistance.
For SNNGP
- Internal nucleophile must be present
- Internal nucleophile must be anti to lg.
During NGP:
(enantiomers)
+ (enantiomer) Rate 3 > 2 > 1
|
334 videos|656 docs|300 tests
|
FAQs on Chemical Reaction: Nucleophilic Substitution Reactions - Chemistry for JEE Main & Advanced
| 1. What is a nucleophilic substitution reaction? |  |
| 2. What is the difference between SN1 and SN2 reactions? |  |
| 3. What is the role of neighboring group participation (NGP) in nucleophilic substitution reactions? |  |
| 4. Can you provide an example of a nucleophilic substitution reaction? |  |
| 5. What factors influence the mechanism of nucleophilic substitution reactions? |  |






















