Class 10 Science: CBSE Sample Question Paper (2020-21) - 2 | CBSE Sample Papers For Class 10 PDF Download
Class-X
Science Theory
TIME: 3 Hrs.
M.M: 80
General Instructions:
Read the following instructions very carefully and strictly follow them :
1. The question paper comprises four sections A, B, C and D. There are 36 questions in the question paper.
All questions are compulsory.
2. Section-A - Question no. 1 to 20 - all questions and parts thereof are of one mark each. These questions contain multiple-choice questions (MCQs), very short answer questions and assertion - reason type questions. Answers to these should be given in one word or one sentence.
3. Section-B - Question no. 21 to 26 are short answer type questions, carrying 2 marks each. Answers to these questions should be in the range of 30 to 50 words.
4. Section-C - Question no. 27 to 33 are short answer type questions, carrying 3 marks each. Answers to these questions should be in the range of 50 to 80 words.
5. Section-D - Question no. - 34 to 36 are long answer type questions carrying 5 marks each. Answers to these questions should be in the range of 80 to 120 words.
6. There is no overall choice. However, internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
7. Wherever necessary, neat and properly labelled diagrams should be drawn.
SECTION A
Q.1.
This reaction can be classified as (1 Mark)
(A) Combination reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
Which of the following is the correct option?
Q.2. Why does the Sun appear white at noon? (1 Mark)
Ans. The light is least scattered at noon.
Q.3. The image formed by a concave mirror is observed to be real, inverted and larger than the object. Where is the object placed? (1 Mark)
Ans. Between the principal focus and the centre of curvature.
OR
Name the part of a lens through which a ray of light passes without suffering any deviation.
Ans. Optical centre
Q.4.
Assertion: After whitewashing the walls, a shiny white finish on the walls is obtained after two to three days.
Reason: Calcium Oxide reacts with Carbon dioxide to form Calcium Hydrogen Carbonate which gives a shiny white finish.
Q.5. On which factor does the colour of the scattered white light depend? (1 Mark)
Ans. Size of the particles of the medium through which it is passing.
Q.6. What is a homologous series of carbon compounds? (1 Mark)
Ans. A homologous series is the family of an organic compound having the same functional group and the successive (adjacent) members of which differ by CH2 unit or 14 mass unit.
Q.7. State two physical properties of gold which are of extreme use to jewellers. (1 Mark)
Ans. Malleability, ductility, lustrous.
Q.8.
Q.9.
(i) It turns lime water milky
(ii) It extinguishes a burning splinter
(iii) It dissolves in a solution of sodium hydroxide
(iv) It has a pungent odour
OR
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) High melting point
Q.11.
Grass → Deer → Tiger
Assertion (A): To dilute concentrated sulphuric acid water is added to the acid slowly.
Reason (R): A lot of heat energy will be given out in the dilution of concentrated sulphuric acid
Assertion (A): Two bar magnets attract when they are brought near to each other with the same pole.
Reason (R): Unlike poles, they will attract each other.
Assertion (A): Double fertilisation is unique to angiosperms.
Reason (R): Triple fusion occurs in asexual reproduction.
Directions: Q. No 17 - 20 contain five sub-parts each. You are expected to answer any four subparts in these questions. (1 Mark)
Q.17. Read the given passage and answer any four questions.
The physical states of the reactants and products can be represented by using the symbols, (s) for solids, (l) for liquids, (g) for gases and (aq) for an aqueous solution along with their respective formulae. The word aqueous is written if the reactant or product is present as a solution in water. The precipitate can also be represented by using an arrow pointing downwards (↓) instead of using the symbol (s). In the same way, the gaseous state of an evolved gas can be represented by using an arrow pointing upward direction (↑) instead of using the symbol (g). The specific condition of the reaction like temperature, pressure, catalyst etc. is written above or below the arrow in the chemical equation.
Magnesium reacting with dil. sulphuric acid
2Na (s) + 2H2O (l) → 2NaOH (X) + H2 (Y)
Q.18. Read the given passage and answer any four questions. (1 Mark)
Sanjana is suffering from frequent stomach pain and vomiting. She went to the Doctor. The doctor asked her to go for an ultrasound. In the report, a stone was found in her gall bladder. Doctor asked her to remove the gall bladder by the operation. But she was reluctant to go for the operation.
Q.19. Read the following and answer any four questions. (1 Mark)
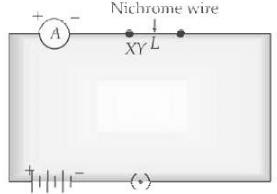
In the given circuit, connect a nichrome wire of length ‘L’ between points X and Y and note the ammeter reading.
(e) ‘‘Potential difference between points A and B in an electric field is 1 V’’. Explain the above statement.
Ans. It means that 1 J of work is being done to move a charge of 1 coulomb from point A to point B.
Q.20. Study the following food web and answer any four questions. (1 Mark)

SECTION B
Q.21. Bile juice does not have any digestive enzyme but still plays a significant role in the process of digestion. Justify the statement. (2 Mark)
Ans. Bile juice makes acidic food coming from the stomach alkaline for the action of pancreatic enzymes. Bile salts break the large globules of fat in the intestine into smaller globules increasing the efficiency of enzyme action. This is similar to the emulsifying action of soaps on dirt.
Q.22. List four characteristics of the image formed by plane mirrors. (2 Mark)
Ans. Four characteristics of the image formed by plane mirror :
(i) It is always virtual and erect.
(ii) Size of the image is equal to that of the object.
(iii) Image is formed at the same distance behind the mirror as the object is in front of the mirror.
(iv) Image is laterally inverted.
Q.23. What is DNA copying? State its importance. (2 Mark)
Ans. DNA replication or DNA copying is the process of producing two identical replicas from one original DNA molecule during cell division.
Importance of DNA Copying :
(i) DNA replication needs to occur so that during cell division, new cells will also have a copy of the organism’s DNA.
(ii) DNA is necessary to make all the RNA and proteins needed for cells to carry out necessary reactions and cellular processes in order to survive.
OR
What are genes? Where are the genes located?
Ans. Genes are basic units of heredity. They are linear segments of DNA that code for a gene product. Genes are located on chromosomes.
Q.24. Write two main differences between an acid and a base? (2 Mark)
Ans.
Q.25. Fresh milk has a pH of 6. When it changes into curd (Yogurt), will its pH value increases or decreases? Why? (2 Mark)
Ans. When milk changes into curd, its pH will decrease. 1 Because curd contains lactic acid, so H+ ion concentration increases and thus pH will decrease.
Q.26. State Mendeleev’s periodic law. Write two achievements of Mendeleev’s periodic table. (2 Mark)
Ans. Mendeleev’s Periodic Law: “Properties of elements are the periodic function of their atomic masses.” Achievements :
(i) It could classify all the elements discovered at that time.
(ii) It helped in the discovery of new elements.
(iii) It helped in the correction of the atomic mass of some of the elements.
OR
State the modern periodic law for the classification of elements. How many (a) groups and (b) periods are there in the modern periodic table?
Ans. “Properties of elements are the periodic function of their atomic number.”
(i) There are 18 groups.
(ii) There are 7 periods in the modern periodic table.
SECTION C
Q.27. After self-pollination in pea plants with round, yellow seeds, the following types of seeds were obtained by Mendel : (3 Mark)
Analyse the result and describe the mechanism of inheritance that explains these results.
Ans. The ratio obtained is 9:3:3:1 in which parental as well as new combinations are observed. This indicates that progeny plants have not inherited the whole set of genes from each parent.
Every germ cell takes on a chromosome from the pair of maternal and paternal chromosomes. When two germ cells combine, the segregation of one pair of characters is independent of the other pair of characters.
Q.28. 2 ml of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test tube. When the contents are warmed, a gas evolves which is bubbled through a soap solution before testing. Write the equation of the chemical reaction involved and the test to detect the gas. Name the gas which will be evolved when the same metal reacts with the dilute solution of a strong acid? (3 Mark)
Ans. Zn + 2NaOH → Na2ZnO2 + H2
When a burning splinter is brought near the gas, it burns with a Pop Sound.
Gas – Hydrogen / H2
Q.29. Explain where and how urine is produced? (3 Mark)
Ans. ➤ Blood passes through filtration units in the kidney called the nephron.
➤ Passes through glomerulus in the Bowman's capsule - Ultrafiltration
➤ Filtrate initially has glucose, amino acids, water, salts and nitrogenous waste
➤ Reabsorption – Water (as per the need of the body), Glucose and amino acids are all reabsorbed.
➤ Secretion of excess water, salts and urea (nitrogenous waste), which makes up the urine.
Q.30. Write the functions of the following parts of the human female reproductive system :
(i) Ovary, (ii) Fallopian tube, (iii) Uterus. (3 Mark)
Ans. (i) Ovary: Produces egg or female gamete, female sex hormone/estrogen.
(ii) Fallopian tube: Transfer of ovum to the uterus, site for fertilization
(iii) Uterus: Site of implantation of the zygote, development of the embryo.
Q.31. Draw a ray diagram to show the path of the reflected ray in each of the following cases. A ray of light incident on a convex mirror. (3 Mark)
(i) Strikes at its pole making an angle θ from the principal axis.
(ii) Is directed towards its principal focus.
(iii) Is parallel to its principal axis.
Ans.
(i)
(ii)
(iii)
Q.32. (a) Explain the formation of Calcium Chloride with the help of electron dot structure. (Atomic numbers: Ca = 20; Cl = 17)
(b) Why do ionic compounds not conduct electricity in the solid-state but conduct electricity in a molten and aqueous state? (3 Mark)
Ans. (a)

(b) Ionic compounds do not conduct electricity in a solid-state due to the absence of free ions but they conduct electricity in the molten and aqueous state due to the presence of free ions.
Q.33. What is a solenoid? Draw the pattern of magnetic field lines of (i) a current-carrying solenoid and (ii) a bar magnet. List two distinguishing features between the two fields? (3 Mark)
Ans. A coil of many turns of insulated copper wire wrapped closely in the shape of a cylinder.
(i)
(ii)
Distinguishing features :

SECTION D
Q.34. Match the following pH values 1, 7, 10, 13 to the solutions given below: (5 Mark)
- Milk of magnesia
- Gastric juices
- Brine
- Aqueous Sodium hydroxide.
Amit and Rita decided to bake a cake and added baking soda to the cake batter.
Explain with a balanced reaction, the role of baking soda. Mention any other use of baking soda.
Ans.
- Milk of magnesia: 10
- Gastric juices: 1
- Brine: 7
- Aqueous sodium hydroxide: 13
Baking soda undergoes thermal decomposition to form Na2CO3, CO2 and H2O; CO2 makes the cake fluffy & soft.
Uses :
Used in fire extinguishers/ antacid to neutralize excess acid in stomach / to neutralize the effect of acid in insect sting.
OR
(i) Four samples A, B, C and D change the colour of pH paper or solution to Green, Reddish-pink, Blue and Orange. Their pH was recorded as 7, 2, 10.5 & 6 respectively. Which of the samples has the highest amount of Hydrogen ion concentration? Arrange the four samples in the decreasing order of their pH.
(ii) Rahul found that the Plaster of Paris, which he stored in a container, has become very hard and lost its binding nature. What is the reason for this? Also, write a chemical equation to represent the reaction taking place.
(iii) Give anyone use of Plaster of Paris other than for plastering or smoothening of walls.
Ans. (i) (a) B
(b) C, A, D, B
(ii) Due to moisture in the atmosphere it converted into gypsum
CaSO4.0.5H2O + 1½ H2O → CaSO4.2H2O
(iii) Making toys/dolls or statues /fixing broken limbs/making decorative materials.
Q.35. Mention the organ and site of photosynthesis in green plants. What are the raw materials essential for this process? How are they obtained? Write the complete balanced chemical equation for the process. Name the by-products. (5 Mark)
Ans. Photosynthesis takes place in the grana and stroma of the chloroplast (Plastid) in green parts of plants. The raw materials required for this process are carbon dioxide and water in the presence of sunlight and chlorophyll. Carbon dioxide enters the leaves through stomata and cells of the roots absorb water from the soil. The balanced equation for photosynthesis :

The by-products in this process are the evolution of oxygen gas.
OR
(i) Draw the diagram of a cross-section of a leaf and label the following parts :
(a) chloroplast
(b) cuticle
(ii) A gas is released during photosynthesis. Name the gas and also state the way in which the gas is evolved.
(iii) In a certain group of plants, stomata remain closed during the day. How is food synthesized by such plants? Also, name them.
Ans.
(i) (ii) Oxygen
(ii) Oxygen
By splitting of water (photolysis)
(iii) They take up CO2 at night through stomata, which open during the night and produce an intermediate organic acid which is acted upon by the energy absorbed by chlorophyll during the day and breaks up to release CO2. The CO2 so produced internally is used in photosynthesis during the day when stomata are closed. Desert plants. [Xerophytic CAM plants]
Q.36. PQ is a current-carrying conductor in the plane of the paper as shown in the figure below.
(i) Find the directions of the magnetic fields produced by it at points R and S?
(ii) Given r1 > r2, where will the strength of the magnetic field be larger? Give reasons.
(iii) If the polarity of the battery connected to the wire is reversed, how would the direction of the magnetic field be changed?
(iv) Explain the rule that is used to find the direction of the magnetic field for a straight current-carrying conductor.
Ans. (i) The magnetic field lines produced is into the plane of the paper at R and out of it at S.
(ii) Field at S > Field at P Magnetic field strength for a straight current-carrying conductor is inversely proportional to the distance from the wire.
(iii) The current will be going from top to bottom in the wire shown and the magnetic field lines are now in the clockwise direction on the plane which is perpendicular to the wire carrying current.
(iv) Right-hand thumb rule. The thumb is aligned to the direction of the current and the direction in which the fingers are wrapped around the wire will give the direction of the magnetic field.
|
303 docs|7 tests
|
FAQs on Class 10 Science: CBSE Sample Question Paper (2020-21) - 2 - CBSE Sample Papers For Class 10
| 1. What is the CBSE sample question paper for Class 10 Science? |  |
| 2. How can I access the CBSE sample question paper for Class 10 Science? |  |
| 3. Are the CBSE sample question papers for Class 10 Science helpful for exam preparation? |  |
| 4. Can I rely solely on the CBSE sample question papers for Class 10 Science for my exam preparation? |  |
| 5. How can I make the most of the CBSE sample question papers for Class 10 Science? |  |

|
Explore Courses for Class 10 exam
|

|

















