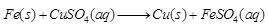Ques 1: When n resistors of equal resistance R each are connected in series, what will be the equivalent resistance?
Ans: When n resistors of equal resistance R each are connected in series, the equivalent resistance becomes R.
Ques 2: Why should the key be taken out after taking the readings?
Ans: The key should be taken out after taking the reading to
(i) avoid heating of resistor wire.
(ii) avoid change in resistivity of wire.
Ques 3: What is the SI unit of potential difference?
Ans: SI unit of potential difference is volt.
Ques 4: What is nuclear fusion?
Ans: Nuclear Fusion Reaction in which two or more small nuclei fuse to form a heavy nucleus is called nuclear fusion, e.g., fusion of hydrogen.
Ques 5: Where does dark reaction of photosynthesis occur?
Ans: Dark reaction of photosynthesis occurs in stroma.
Ques 6: The leaf of a destarched plant is covered with black paper strip. After 8 h, starch test is done. In which part will the colour be blue-black?
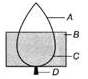
Ans: In 'A' part, the colour will be blue-black. It is because, photosynthesis occurred at uncovered portion results in the formation of starch which gives blue-black colour with starch test i.e., iodine solution.
Ques 7: Name the process by which roots absorb water from soil.
Ans: Osmosis is the process by which roots absorb water from soil.
Ques 8: Why is tungsten metal selected for making filaments of incandescent lamp bulb?
Ans: Tungsten has a high melting point. Therefore, it is used for making filament of bulbs.
Ques 9: Which is the largest gland of our body and what is its function?
Ans: Liver is the largest gland of our body and it secretes bile juice for digestion of fat.
Ques 10: Compare the pH of oxalic acid with seven.
Ans: The pH of oxalic acid is less than seven.
Ques 11: A blue litmus paper was first dipped in dil. HC1 and then in dil. NaOH solution. What change in colour of litmus paper you will observe?
Ans: A blue litmus paper was first dipped in dil. HC1 and then in dil. NaOH solution. The colour of litmus paper became first red and then blue.
Ques 12: When zinc granules are kept in copper sulphate solution, what change in colour of solution you will observe?
Ans: When zinc granules are kept in copper sulphate solution, the colour of solution becomes colour less due to the formation of
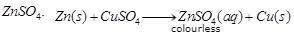
Ques 13: (a) When do we put the resistance in series combination?
(b) How will you find the equivalent resistance of two resistors when they are connected in series?
Ans: (a) When we have the smaller value of resistance and need the greater value of resistance, we put them in series combination.
(b) The equivalent resistance of two resistors R1 and R2 is determined by Rs = R1 + R2
Ques 14: (a) Four students were asked to test the pH of four samples as shown under. Whose result is reported correctly?
| Student | H2O | CH2COOH | HCl | NaOH |
| A | 7 | 1 | 1 | 1 |
| B | 7 | 3 | 1 | 1 |
| C | 7 | 1 | 1 | 13 |
| D | 7 | 3 | 1 | 13 |
(b) Bottle A contains a dilute solution of vinegar and bottle B contains sodium carbonate solution. Write the colour seen in pH paper dipped in A and B.
Ans: (a) The result of student D. It is because, water is neutral having pH = 7 CH3 COOH is a weak acid having pH = 3 HCl is a strong acid having pH = 1 NaOH is strong base having pH =13 (b) In bottle A colour of pH paper is orange due to the presence of acid (vinegar). In bottle B colour of pH paper is blue due to the presence of base (sodium carbonate solution).
Ques 15: Which mechanism plays an important role in transportation of water in plants?
(a) During daytime
(b) At night
Ans: (a) During daytime transpiration pull plays an important role.
(b) At night root pressure plays an important role.
Ques 16: In the figure given below
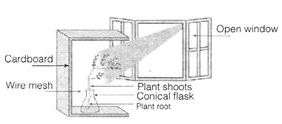
(a) Which type of movement is shown by the plant in above activity?
(b) The information below shows the function of the responses of plants To gain mineral salts in the soil and to get support. Name the response related with the functions above.
Ans: (a) Photo tropic movement is shown by the plant because its shoot bends in the direction of light.
(b) At the above functions like, to gain mineral salts in the soil and to get support are done by roots. These roots grow towards the gravity. Therefore, the response related to the above function is due to gravity i.e., positive geotropism.
Ques 17: Name the green dot-like structures in some cells observed by a student when a leaf-peel was viewed under a microscope. What is this green colour due to?
Ans: The green dot-like structures are chloroplasts. This green colour is due to the presence of a green pigment called chlorophyll. Chlorophyll is present in cell organelles called chloroplast.
Ques 18: Write chemical formula for calcium oxychloride. How is it prepared? What is its common name? What is its use?
Ans: Chemical formula for Calcium oxychloride is CaOCl2. Its common name is bleaching powder. It is prepared by the action of chlorine on dry slaked lime.
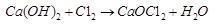
It is used in paper industry to bleach wood pulp.
Ques 19: A non-metal A which is the largest constituent of air, when heated with H2 in 1:3 ratio in the presence of catalyst (Fe) gives a gas B. On heating with O2 it gives anoxide C. If this oxide is passed into water in the presence of air, it gives an acid D which acts as a strong oxidizing agent.
(a) Identify A, B, C and D.
(b) To which group of the Periodic Table does this non-metal belongs?
Ans: (a) A is nitrogen (N) (because in air, percentage of nitrogen is 78%). When it reacts with H2, it forms NH3.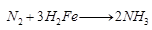 Thus, B is ammonia (NH3). When it is heated with O2 it forms NO2.
Thus, B is ammonia (NH3). When it is heated with O2 it forms NO2.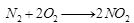 C is NO2 (nitrogen dioxide). When it is passed into H2O, it forms HNO3. Thus, D is nitric acid (HNO3).
C is NO2 (nitrogen dioxide). When it is passed into H2O, it forms HNO3. Thus, D is nitric acid (HNO3).
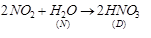
(b) The non-metal belongs to group 15 or VA.
Ques 20: How would you distinguish between baking soda and washing soda by heating?
Ans: On heating NaHCO3 (baking soda), (carbon dioxide) gas is given out that turns lime water milky.
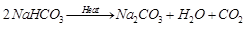
While on heating Na2CO3.10H2O (washing soda), water of crystallization is given out and the salt becomes anhydrous. The presence of water of crystallization given as product can be tested by treating it with anhydrous CuSO4 (white) which becomes blue in colour in its contact.
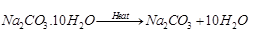
Ques 21: An electric heater is rated at 2kW Calculate the cost of using it for 2 hours daily for the month of September, if each unit costs Rs. 4.00.
Ans: Electric energy consumed by heater in one day
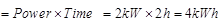
Electric energy consumed by heater in one month = 4 x 30 = 120 kWh
∴ September = 30 days] = 120 units
[∴1 unit = 1 kWh]
Cost of using the heater = 120 x 4.00 = Rs. 480.00
Ques 22: Define electric power. An electric motor takes 5 A from a 220 V line. Find the power of the motor and energy consumed in 2 h.
Ans: The electrical energy consumed by a device per unit time is called electric power.
Given, Current (I) = 5A, Potential difference (V) = 220V, Time (t) = 2h Power of electric motor (P) = VI = 220 x 5 = 1100 W = 1.1 kW
Energy consumed by the motor = Pt = 1.1 kW x 2h = 2.2 kWh
Ques 23: How does transpiration help in the functioning of plant? A plant leaf was coated with Vaseline. What happens to
Ans: Transpiration is important in plant because
(i) It helps in ascent of sap through xylem.
(ii) It helps in removal of excessive water.
(iii) It helps in temperature regulation of plant. Leaf does not remain healthy when coated with vaseline, because vaseline coat does not allow gaseous exchange and transpiration from leaf surface.
Ques 24: (a) On what factors, the resistance of the conductor depends?
(b) What is (i) the highest, (ii) the lowest resistors that can be secured by combinations of four coils of resistance 4Ω, 8Ω, 12Ω, 24Ω?
Ans: (a) Resistance of a conductor depends on the following factors (i) Resistance of a conductor is directly proportional of the length of the conductor i.e., R ∝ l.
(ii) Resistance of a conductor is inversely proportional to the area of cross-section. 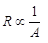
(b) (i) The overall resistance is increased in a series combination. ∴ Highest equivalent resistance = R1 + R2 + R3 + R4 = 4Ω + 8Ω + 12Ω + 24Ω + 48Ω
(ii) The overall resistance is reduced in a parallel combination. ∴ Lowest equivalent resistance 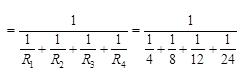 = 1/12 = 2Ω
= 1/12 = 2Ω
Ques 25: What are the functions of nervous system? Give two parts of vertebrate nervous system.
Ans: Nervous system forms a network in the body for regulation, coordination and control of activities and functions of body. There are two parts of vertebrate nervous system.
(i) Central Nervous System (CNS) includes spinal cord and brain.
(ii) Peripheral Nervous System (PNS) includes cranial nerves, visceral nerves and spinal nerves.
Ques 26: A copper coil is connected to a galvanometer. What would happen if a bar magnet is
(a) pushed into the coil with its North pole entering first?
(b) held at rest inside the coil?
(c) pulled out again?
Ans: (a) When North pole is pushed into the coil, a momentary deflection is observer in the galvanometer. This deflection indicates that a momentary current is produced in the coil. The direction of current in the coil is anti-clockwise.
(b) When the magnet is held at rest, there is no deflection in the galvanometer. It indicates that no current is produced in the coil in this use.
(c) In pulling the magnet out of the coil, a deflection in opposite direction is observed. It indicates that the current produced in the coil is in opposite direction.
Ques 27: How is biogas produced? Why is it a better fuel than cow dung?
Ans: Biogas is produced from biomass when it decays in the absence of oxygen in presence of bacteria in a biogas plant. It is a better fuel than cow dung cake because
(i) It is a clean fuel, produces no smoke residue.
(ii) It has high calorific value.
(iii) It helps in waste disposal, even the left over slurry is used as manure.
Ques 28: (a) An iron nail is kept in copper sulphate solution. What happens and why?
(b) A metal M does not liberate hydrogen from acids but reacts with oxygen to give a black colour product. Identify M and black coloured product and also explain the reaction of M with oxygen.
Ans: (a) If an iron nail is kept in CuSO4 solution, then iron nail gets brown coating due to Cu deposition and the? Solution turns greenish due to the formation of FeSO4.
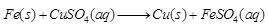
It is because, Fe being more reactive than Cu, displaces Cu from CuSO4 solution.
(b) M is copper (Cu) as it is less reactive than hydrogen and gives. black product, copper oxide (CuO) with oxygen.
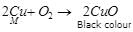
Ques 29: Malini stores curd in a beautifully designed copper containers for a party in the evening. The guests complain that curd has turned bitter. She gets embarrassed and feels sorry. One of the guests told her why this happens, so that she would careful next time.
(a) What suggestion would have been
(b) List values of Malini.
(c) What values are shown by the guest?
Or
Manisha is experimenting with acids in the lab. She is suggested by Nisha to wear lab coat and goggles which she ignores. The acid spills over her dress and burns her hand too. Nisha immediately rescues her by providing first aid and takes her to medical room.
(a) Throw light on values of Nisha.
(b) What precautions should be taken, while working in laboratory?
(c) Give examples of some strong acids.
Ans: (a) He suggested her not to store curd in metal container as lactic acid present in curd forms salts with metal which taste bitter.
(b) Malini has a good aesthetic sense, generous, polite and courteous.
(c) The guest is intelligent, practical, sharing and convincing.
Or
(a) Nisha is caring, spontaneous, cautious and helpful.
(b) In lab, one should wear lab coat and goggles. No chemical should be touched or tasted. Apparatus and materials should be handled with most care.
(c) Some strong acids are H2SO4, HCl, HNO3, etc
Ques 30: Rohit's father told him that various pipes are responsible for transport of blood in our body. But he thinks that these are of single type. Describe the types of blood vessels present in our body. Explain the function of each to help Rohit. Or Hemant says his grandmother that water and minerals are transported from root to leaf in plant. To help his grandmother, explain the mechanism of water and minerals transport in plant.
Ans: Three types of blood vessels are connected to form a closed system in human body. (i) Arteries These are wide, thick elastic walled vassels, which carry blood from heart to all body parts. Arteries carry oxygenated blood except pulmonary artery, which carries deoxygenated blood from heart to lungs.
(ii) Capillaries Arteries keep dividing further into smaller vessels and finally for the smallest vessels called capillaries. These have permeable wall and exchange gases, nutrients and waste products across the tissues.
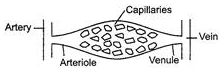
(iii) Veins The capillaries combine together to form thin walled vessels called veins. Veins carry deoxygenated blood from body organs to the heart. Pulmonary vein carries oxygenated blood from lungs to the heart.
Or
The mechanism of transport of water and minerals in plants is as follows (i) The xylem forms an interconnected network of vascular tissues, which create a continuous channel from root tracheids to all plant parts.
(ii) The cells of root hair in contact with soil take up ions, which create an ion concentration gradient between root and soil.
(iii) The water flows into root from soil due to osmosis.
(iv) Stomata loose water to the surroundings through transpiration. This creates a suction force, which pulls water upwards to heights.
(v) The steady movement of water into the root from the soil pushes water upwards.


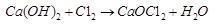
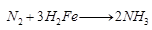 Thus, B is ammonia (NH3). When it is heated with O2 it forms NO2.
Thus, B is ammonia (NH3). When it is heated with O2 it forms NO2.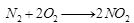 C is NO2 (nitrogen dioxide). When it is passed into H2O, it forms HNO3. Thus, D is nitric acid (HNO3).
C is NO2 (nitrogen dioxide). When it is passed into H2O, it forms HNO3. Thus, D is nitric acid (HNO3).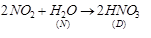
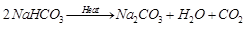
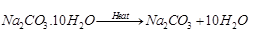
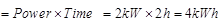
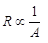
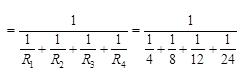 = 1/12 = 2Ω
= 1/12 = 2Ω