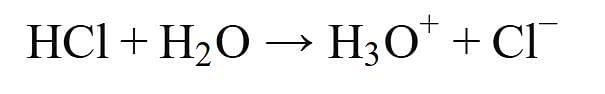Q1:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion: After white washing the walls, a shiny white finish on walls is obtained after two to three days.
Reason: Calcium Oxide reacts with Carbon dioxide to form Calcium Hydrogen Carbonate which gives shiny white finish.
Explanation
-
Assertion (A): After white washing the walls, a shiny white finish on walls is obtained after two to three days.
- This statement is true. After applying whitewash (which typically contains calcium hydroxide, not calcium oxide directly), the walls initially appear wet and chalky. Over time, as the whitewash dries and carbon dioxide from the air reacts with calcium hydroxide, a shiny white finish develops.
-
Reason (R): Calcium Oxide reacts with Carbon dioxide to form Calcium Hydrogen Carbonate which gives shiny white finish.
- This statement is partly incorrect:
Calcium oxide (CaO) reacts with carbon dioxide (CO2) to form calcium carbonate (CaCO3), not calcium hydrogen carbonate. Calcium carbonate can contribute to the shiny white appearance after whitewashing, but it does not form calcium hydrogen carbonate as stated.
Report a problem
Q2:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
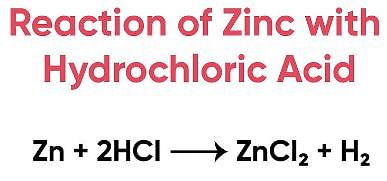
Try yourself:Assertion (A): When zinc is added to dilute hydrochloric acid, hydrogen is given off.
Reason (R): Hydrogen chloride molecules contain hydrochloric acid and hydrogen atoms
Explanation

-
Assertion (A): When zinc is added to dilute hydrochloric acid, hydrogen is given off.
- This statement is true. When zinc metal reacts with dilute hydrochloric acid (HCl), it produces zinc chloride (ZnCl2) and hydrogen gas (H2).
-
Reason (R): Hydrogen chloride molecules contain hydrochloric acid and hydrogen atoms.
- This statement is misleading:
Hydrogen chloride (HCl) is a gas composed of hydrogen and chlorine atoms, not hydrogen atoms alone. It dissociates in water to form hydrochloric acid (HCl). However, this does not directly relate to the reaction of zinc with hydrochloric acid where hydrogen gas is produced due to the displacement reaction between zinc and the hydrogen ions in the acid.
Report a problem
Q3:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion (A): Gas bubbles are observed when sodium carbonate is added to dilute hydrochloric acid.
Reason (R): Carbon dioxide is given off in the reaction.
Explanation
Report a problem
Q4:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion (A): Ammonia solution is an alkali.
Reason (R): Ammonia solution turns blue litmus paper red.
Explanation
Report a problem
Q5:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): When common salt is kept open, it absorbs moisture from the air.
Reason (R): Common salt contains little magnesium chloride.
Explanation
Magnesium chloride present in common salt is a deliquescent substance i.e. it absorbs moisture from the air when kept in open.
Report a problem
Q6:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion (A): Baking soda creates acidity in the stomach.
Reason (R): Baking soda is alkaline.
Explanation
Report a problem
Q7:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion (A): Plaster of Paris is used by doctors for setting fractured bones.
Reason (R): When Plaster of Paris is mixed with water and applied around the fractured limbs, it sets into a hard mass.
Explanation
-
Assertion (A): Plaster of Paris is used by doctors for setting fractured bones.
- This statement is true. Plaster of Paris (calcium sulfate hemihydrate, CaSO4·0.5H2O) is commonly used in medicine for immobilizing broken bones. It is mixed with water to form a paste that sets into a hard mass, providing support and stability to the fractured limb.
-
Reason (R): When Plaster of Paris is mixed with water and applied around the fractured limbs, it sets into a hard mass.
- This statement is true and correctly explains why plaster of Paris is effective in setting fractured bones. When mixed with water, plaster of Paris undergoes an exothermic reaction, forming gypsum (calcium sulfate dihydrate, CaSO4·2H2O), which hardens rapidly into a solid mass.
Report a problem
Q8:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Direction: In the Following Questions, A Statement of Assertion (A) Is Followed by A Statement of Reason (R). Mark The Correct Choice As:
Assertion: While dissolving an acid or base in water, the acids must always be added slowly to water with constant stirring.
Reason: Dissolving an acid on a base in water is a highly exothermic reaction.
Explanation
-
Assertion (A): While dissolving an acid or base in water, the acids must always be added slowly to water with constant stirring.
- This statement is true. When dissolving acids or bases in water, especially concentrated solutions, adding them slowly to water with stirring helps to control the reaction and prevents splashing or excessive heat generation.
-
Reason (R): Dissolving an acid or base in water is a highly exothermic reaction.
- This statement is true. The process of dissolving strong acids or bases in water is exothermic, meaning it releases heat.
Report a problem
Q9:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion : On adding H2SO4 to water the resulting aqueous solution gets corrosive.
Reason: Hydronium ions are responsible for corrosive action.
Explanation
-
Assertion (A): On adding H2SO4 to water, the resulting aqueous solution gets corrosive.
- This statement is true. Sulfuric acid (H2SO4) is a strong acid that dissociates completely in water to form hydronium ions (H3O+) and sulfate ions (SO4^2-). The resulting solution is highly acidic and corrosive to many materials.
-
Reason (R): Hydronium ions are responsible for corrosive action.
- This statement is true and correctly explains why the solution becomes corrosive. Hydronium ions (H3O+) in acidic solutions have a high affinity for reacting with materials, causing corrosion or damage to surfaces they come into contact with.
Report a problem
Q10:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion : Phenolphthalein gives pink colour in basic solution.
Reason : Phenolphthalein is a natural indicator.
Explanation
Report a problem
Q11:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion: HCl gas does not change the colour of dry blue litmus paper.
Reason: HCl gas dissolves in the water present in wet litmus paper to from H+ ions.
Explanation
Assertion:
"HCl gas does not change the colour of dry blue litmus paper."
- This statement is true. Dry HCl gas cannot release hydrogen ions (H⁺) because it needs to be dissolved in water to form hydronium ions (H3O+H_3O^+H3O+), which cause the acidic effect. Therefore, HCl gas will not change the colour of dry blue litmus paper.
Reason:
"HCl gas dissolves in the water present in wet litmus paper to form H⁺ ions."
- This statement is also true. When HCl gas dissolves in the water present on wet litmus paper, it dissociates to form H⁺ ions, which can then change the colour of blue litmus to red, indicating an acidic environment.
Conclusion:
Both the assertion and reason are true, and the reason correctly explains the assertion.
Thus, the correct answer is:
Option 1: Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Report a problem
Q12:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion : HCl produces hydronium ions (H3O+) and chloride ions (Cl-) in aqueous solution.
Reason : In presence of water, bases give H+ ions.
Explanation
-
Assertion (A): HCl produces hydronium ions (H3O+) and chloride ions (Cl-) in aqueous solution.
- This statement is true. When hydrogen chloride (HCl) dissolves in water, it dissociates completely into hydronium ions (H3O+) and chloride ions (Cl-). The reaction can be represented as:

-
Reason (R): In presence of water, bases give H+ ions.
- This statement is false. Bases do not give H+ ions in water; they give OH- ions instead. The presence of OH- ions characterizes a basic solution, not H+ ions.
Report a problem
Q13:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion: Sodium hydroxide reacts with zinc to produce hydrogen gas.
Reason : Acids react with active metals to produce hydrogen gas.
Explanation
Assertion (A): Sodium hydroxide reacts with zinc to produce hydrogen gas.
- This statement is true. When sodium hydroxide (NaOH) reacts with zinc (Zn), it produces hydrogen gas (H2).
Zn (s) + 2NaOH (aq) → Na2ZmO2 (aq)+ H2 (g)
Reason (R): Acids react with active metals to produce hydrogen gas.
- This statement is also true. Acids such as hydrochloric acid (HCl) or sulfuric acid (H2SO4) react with active metals like zinc (Zn) to produce hydrogen gas.
Report a problem
Q14:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion : Ammonia solution is an alkali.
Reason : Ammonia solution turns blue litmus paper red.
Explanation
Report a problem
Q15:
Question for Assertion & Reason Type Questions: Acids, Bases and Salts
Try yourself:Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.
Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.
Explanation
Reason (R) : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid. Ans. Correct option : (d) Explanation: Water is never added to concentrated sulphuric acid as it is an exothermic reaction and releases a large amount of heat energy.
Report a problem