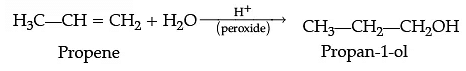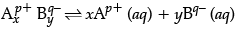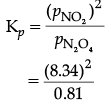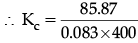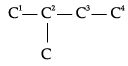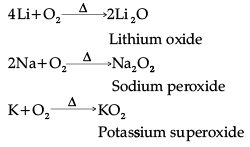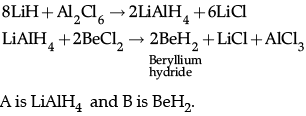Class 11 Chemistry: CBSE Sample Question Papers- Term II (2021-22)- 1 | Sample Papers for Class 11 Medical and Non-Medical - JEE PDF Download
Class : XI
Time : 120 Minutes
Maximum Marks : 40
General Instructions:
- There are 12 questions in this question paper with internal choice.
- Section A: Q. No. 1 to 3 are very short answer questions carrying 2 marks each.
- Section B: Q. No. 4 to 11 are short answer questions carrying 3 marks each.
- Section C: Q. No. 12 is case based question carrying 5 marks.
- All questions are compulsory. 6. Use of log tables and calculators is not allowed.
Section - A
Q.1. Arrange the following in the increasing order of their property indicated (Any 2):
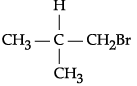
(i) 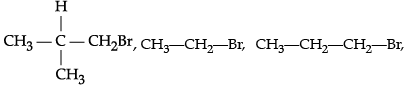 CH3Br (rate of β-elimination reaction with alcoholic KOH)
CH3Br (rate of β-elimination reaction with alcoholic KOH)
(ii) n-butane, 2-methylbutane, n-pentane, 2,2-dimethylpropane (boiling points)
(iii) H2C=CH2, (CH3)2C = CH2 ,CH3—CH= CH2, CH3 —CH= CH —CH2 — CH3 (electrophilic addition reaction)
(i) CH3Br does not have any β-carbon so it would not show any reaction.
More the number of β-substituents (alkyl groups), more stable alkene will be formed on β-elimination and more will be the reactivity. Thus, the increasing order of reactivity of the rate of β- elimination with alc. KOH is
CH3Br < CH3—CH2—Br < CH3—CH2—CH2—Br <(ii) n-butane < 2,2-dimethylpropane < 2-methylbutane < n-pentane.
As the number of carbon atoms in the chain increases, boiling point increases. Boiling point decreases with increase in branching.
(iii)The stability of the intermediate carbocation determines the reactivity towards electrophilic addition reactions. Due to the inductive effect (+I) of alkyl group, the bigger is the attached alkyl group, the more stable is the intermediate ion. Thus, the order of stability of carbocation is:
Primary carbocation(1°) < Secondary carbocation (2°) < Tertiary carbocation (3°).
Hence, the order of reactivity of the above alkenes towards electrophilic addition reactions increases in the order:
H2C=CH2, < CH3 —CH= CH2 <CH3—CH=CH—CH2—CH3 <(CH3)2C= CH2
Q.2. What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30°C?
Given,
P1 = 1 bar
P2 = ?
V1= 500 dm3
V2= 200 dm3
Temperature remains constant at 30°C.
∴ According to Boyle’s law,
P1V1 = P2V2
1 × 500 = P2 × 200
P2= 2.5 bar
Q.3. Give reasons to support your answer:
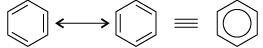
(i) Benzene is extra ordinarily stable though it contains three double bonds.
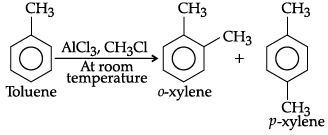
(ii) Toluene on Friedel-Crafts methylation gives o– and p–xylene.
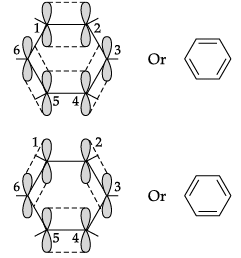
(i) Benzene is a hybrid of resonating structures given as:
All six carbon atoms in benzene are sp2 hybridised. The two sp2 hybrid orbitals of each carbon atom overlap with the sp2 hybrid orbitals of adjacent carbon atoms to form six sigma bonds in the hexagonal plane. The remaining sp2 hybrid orbital on each carbon atom overlaps with the s-orbital of hydrogen to form six sigma C–H bonds. The remaining unhybridised p-orbital of carbon atoms has the possibility of forming three π bonds.
The six π-electrons are delocalised and can move freely about the six carbon nuclei. Even after the presence of three double bonds, these delocalised π-electrons stabilise benzene.
(ii) Methyl (-CH3) group attached to benzene ring increases electron density to benzene ring at ortho and para-positions due to its electron releasing nature (i.e., + R effect), So, tolueneon Friedel- Crafts methylation gives o-and p-xylene.
Section - B
Q.4. Account for the following:
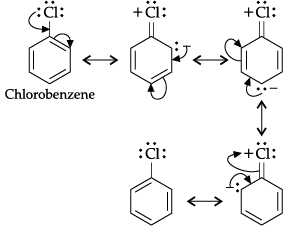
(i) Benzene undergo electrophilic substitution reactions easily but nucleophilic substitutions with difficulty.
(ii) Rotation around carbon-carbon single bond of ethane is not completely free.
(iii)Halogens are o- and p-directing in haloarenes despite their -I effect.
OR
How will the following conversions take place? Give chemical reaction of each of the following.
(i) Ethyne into benzene.
(ii) Propene to propan-1-ol.
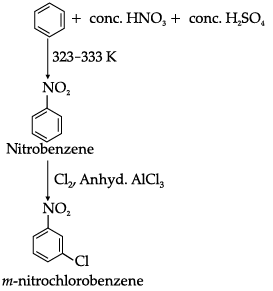
(iii) Benzene to m-nitrochlorobenzene
(i) Benzene is a planar molecule having delocalised electrons above and below the plane of ring. Hence, it is electronrich compound. As a result, it is highly attractive towards electron deficient species i.e., electrophiles. Therefore, it undergoes electrophilic substitution reactions very easily. Nucleophiles are electronrich in nature. Hence, they are repelled by nucleophilic character of benzene. Hence, benzene undergoes nucleophilic substitutions with difficulty.
(ii) Ethane contains carbon-carbon sigma (σ) bond. Electron distribution of the sigma molecular orbital is symmetrical around the internuclear axis of the C–C bond which is not disturbed due to rotation about its axis. This permits free rotation around a C–C single bond. However, rotation around a C–C single bond is not completely free. It is hindered by a small energy barrier due to weak repulsive interaction between the adjacent bonds. Such a type of repulsive interaction is called torsional strain. From all the conformations of ethane, the staggered form has the least torsional strain and the eclipsed form has the maximum torsional strain. The energy difference between the two extreme forms is of the order of, 12.5 kJ mol–1, which is very small. It has not been possible to separate and isolate different conformational isomers of ethane.
(iii) In case of aryl halides, halogens are little deactivating because of their strong –I effect. Therefore, overall electron density on the benzene ring decreases. In other words, halogens are deactivating due to –I effect. However, because of the +R-effect, i.e., participation of lone pairs of electrons on the halogen atom with the π-electrons of the benzene ring, the electron density increases more at o- and p-positions than at m-positions. As a result, halogens are o-, p-directing. The combined result of +R-effect and -I-effect of halogens is that halogens are deactivating but o, p-directing.
OR
(i) Benzene from Ethyne:
(ii) Addition of water to propene in presence of few drops of sulphuric acid (catalyst) and peroxide leads to the formation of propan-1-ol.
(iii)Benzene can be converted into m-nitrochlorobenzene as:
Q.5. Answer the following questions:
(i) If two substances are in equilibrium in a reversible reaction and if the concentration of each reactant is reduced to half, then how the equilibrium constant will be affected?
(ii) (a) Fizz is observed when soda water bottle is opened. Why?
(b) For an exothermic reaction, what happens to the equilibrium constant if temperature is increased?
OR
(i) A sparingly soluble salt having general formula 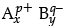 and molar solubility S is in equilibrium with its saturated solution. What will be the relationship between solubility and solubility product of such salt?
and molar solubility S is in equilibrium with its saturated solution. What will be the relationship between solubility and solubility product of such salt?
(ii) (a) State Le-Chatelier ’s principle.
(b) For the given reaction CO(g) + 2H2(g) ⇌ CH3OH(g), ΔrH° = - 92 kJ/mol, predict the direction of the reaction where:
(1) pressure is doubled
(2) temperature is doubled.
(i) The equilibrium constant Keq of a reaction is the ratio of the concentration of products to the concentration of reactants at equilibrium. It is a measure of the extent of the reaction. It is independent of the rate of reaction and initial concentrations of reactants and products but depends on the temperature and pressure. Hence, Keq is not affected by change in concentration of reactants and products.
(ii) (a) An unopened soda water bottle is virtually bubble-free because the pressure inside the bottle keeps the carbon dioxide dissolved in the liquid. When it is opened, pressure is released and the gas bubbles are allowed to wiggle free from the liquid and rise to the surface in the form of fizz.
(b) Equilibrium constant, K = Kf/Kb. In exothermic reaction, with the increase in temperature, equilibrium constant for backward reaction (Kb) increases much more than Kf. Thus, equilibrium constant (K) decreases with increase in temperature for an exothermic reaction.
OR
(i) A sparingly soluble salt having general formulaits molar solubility is S mol L–1
Then,
S moles of AxBy dissolve to give x moles of Ap+ and y moles of Bq–.
Therefore, solubility product
Ksp = [Ap+]x [Bq–]y
= [xS]x [yS]y
= xxyySx+y
(ii) (a) According to Le – Chatelier's principle, “A change in any of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or to counteract the effect of the change”.
(b) CO(g) + 2H2(g) ⇌ CH3OH(g), ΔrH° = – 92 kJ/mol
(1) When pressure is doubled, the equilibrium will shift in the direction where volume decreases i.e. forward direction.
(2) When temperature is doubled, the equilibrium will shift in backward direction because it is an exothermic reaction ΔH <0.
Q.6. Account for the following:
(i) PbCl4 is less stable than SnCl4, but PbCl2 is more stable than SnCl2.
(ii) Carbon shows maximum catenation in Group 14 elements.
(iii)Diamond is covalent, yet it has high melting point.
(i) Stability of +4 oxidation state decreases down the group while that of +2 oxidation state increases due to inert pair effect. Thus, PbCl2 and SnCl4 are more stable than PbCl4 and SnCl2 respectively.
(ii) Due to strong C—C bond, its bond dissociation energy is the highest among Group 14 elements.
(iii)Diamond has three dimensional network structure involving strong C–C bonds. These bonds are difficult to break. Hence, the melting point of diamond is very high.
Q.7. When 13.8 g of N2O4 was placed in a 1L reaction vessel at 400 K and allowed to attain equilibrium, the total pressure at equilibrium was found to be 9.15 bar. Calculate Kc, Kp and partial pressure at equilibrium.
N2O4(g) ⇌ 2NO2(g)
Given,
Total volume (V) = 1L
Mass of N2O4 = 13.8g
Molar mass of N2O4 = 14 × 2 + 16 × 4 = 92g
Number of moles of N2O4(n)= Given mass/Molar mass = 13.8g/92g = 0.15 mol
Gas constant (R) = 0.083 bar L mol–1 K–1
Temperature (T) = 400 K
According to ideal gas equation,
pV = nRT
p × 1 L = 0.15 mol × 0.083 bar L mol–1 K–1 × 400K
∴ p = 4.98 bar
For the given reaction, N2O4(g) ⇌ 2NO2(g)
Initial pressure 4.98 bar 0
At equilibrium (4.98 – x) bar 2x bar
Hence,
ptotal at equilibrium = pN2O4 + pNO2
9.15 = (4.98 – x) + 2x
9.15 = 4.98 + x
∴ x =9.15 – 4.98 = 4.17 bar
Partial pressures at equilibrium are,
pN2O4 =4.98 – 4.17 = 0.81 bar
pNO2 =2x = 2 × 4.17 = 8.34 bar
= 85.87
Kp =Kc (0.083 × 400)1
=2.586 ≈ 2.6
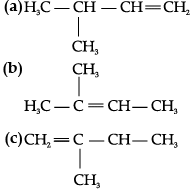
Q.8. Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
The basic skeleton of 2-methylbutane is shown below:
On the basis of this structure, various alkenes that will give 2-methylbutane on hydrogenation are:
Q.9. What happens when following conversion occurs:
(i) Alkali metals are heated in the air.
(ii) When sodium is dissolved in liquid ammonia.
(iii) Metal carbonates are heated.
OR
(i) What is the oxidation state of K in KO2?
(ii) Complete the following reactions: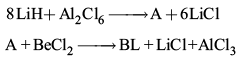
(i) The alkali metals on heating in air form various oxides. Lithium forms monoxide, sodium forms peroxide and potassium forms superoxide.
(ii) Alkali metals like sodium dissolve in liquid ammonia to give deep blue solution forming ammoniated electrons which absorb energy in visible region of light and impart blue colour to the solution.
M +(x+y) NH3 → [M (NH3) x]+ + 2[e (NH3)y]– Ammoniated electron
(iii) Metal carbonates decompose on heating to give metal oxide and carbon dioxide.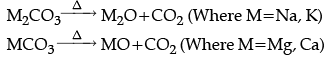
OR
(i) Let, oxidation state of K = x
∴ KO2 contains superoxide ion i.e., O2–
x + (–1) = 0
x – 1 = 0
x = +1∴ The oxidation state of K in KO2 is +1.
(ii)
Q.10. The combustion of one mole of benzene takes place at 298 K and 1 atm. After combustion, CO2(g) and H2O(l) are produced and 3267.0 kJ of heat is liberated. Calculate the standard enthalpy of formation, ∆fH° of benzene. Standard enthalpies of formation of CO2(g) and H2O(l) are – 393.5 kJ mol–1 and – 285.83 kJ mol–1 respectively.
Chemical reaction for the formation of benzene, 6C(graphite,s) + 3H2(g) → C6H6(l), ∆fH° = ? …(i)
Enthalpy of combustion of 1 mol of benzene is :
C6H6(l) + (15/2) O2(g) → 6CO2(g) + 3H2O(l); ∆cH°= – 3267 kJ mol–1 …(ii)
Enthalpy of formation of 1 mol of CO2(g)
C(graphite, s) + O2(g) → CO2(g); ∆fH°= – 393.5 kJ mol–1 …(iii)
Enthalpy of formation of 1 mol of H2O(l) is:
H2(g) + (1/2)O2(g) → H2O(l); ∆fH° = – 285.83 kJ mol–1 …(iv)
Multiplying eq. (iii) by 6 and eq. (iv) by 3, we get
6C(graphite, s) + 6O2(g) → 6CO2(g) ∆fH° = –2361 kJ mol–1 …(v)
3H2(g) + (3/2)O2(g) → 3H2O(l); ∆fH°= – 857.49 kJ mol–1 …(vi)
On adding above equations, (v) and (vi), we get
6C(graphite, s) + 3H2(g) + (15/2)O2(g) → 6CO2(g) + 3H2O(l);
∆f H° = – 3218.49 kJ mol–1 …(vii)
On reversing eq. (ii), we get
6CO2(g) + 3H2O(l) → C6H6(l) + (15/2)O2(g);
∆f H° = 3267 kJ mol–1 …(viii)
On adding equation (vii) and (viii), we get
6C(graphite, s) + 3H2(g) → C6H6(l);
∆fH = 48.51 kJ mol–1
∴ Standard enthalpy of formation of benzene (∆fH°) = 48.51 kJ mol–1
Q.11. (i) Why alkaline earth metals cannot be obtained by reduction of their oxides?
(ii) Why do beryllium and magnesium not impart colour to the flame in the flame test ?
(iii)Among the alkali metals which elements are the most electropositive in nature and lowest in ionic mobility?
OR
On the basis of the figure given below, answer the following questions:
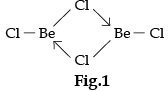
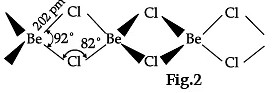
(i) What is the difference between the two structures?
(ii) What would be the physical state of BeCl2 in figure 1 and 2?
(iii) Why does Be not exhibit a coordination number more than four?
(i) The alkaline earth metals cannot be obtained by reduction of their oxides because the enthalpies of formation of these oxides are quite high and consequently they are very stable to heat.
(ii) Beryllium and magnesium do not impart colour to the flame in the flame test due to the small size of Be and Mg atoms. So, these atoms require high excitation energy and are not excited by the energy of the flame.
(iii) Cesium is the most electropositive alkali metal because the attraction of nucleus of cesium is very less towards the outermost electron, so it is likely to lose its its electron, easily to attain stable nable gas configuration.
Lithium ion has the smallest size ion due to more effective nuclear charge. Thus, lithium ion is heavily hydrated that rest element ions of the Group 1. So, Li+ has lowest mobility.
OR
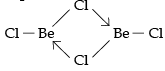
(i) Fig 1 is a bridged structure.
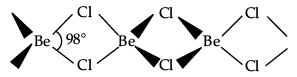
Fig 2 is a chain structure
(ii) Structure of BeCl2 in the vapour state:
BeCl2 has bridged chloride structure in the vapour phase.
Structure of BeCl2 in the solid state:
Beryllium chloride has a chain structure in the solid state.
(iii) Beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the beryllium metal.
Section - C
Q.12. Read the passage given below and answer the questions that follow:
Hydrocarbons are of different types. Saturated hydrocarbons contain carbon-carbon and carbonhydrogen single bonds. Unsaturated hydrocarbons contain carbon-carbon multiple bonds – double bonds, triple bonds or both. Aromatic hydrocarbons are a special type of cyclic compounds that are also known as ‘arenes’. Most of such compounds were found to contain benzene ring. Now, the name is applied to all the ring systems whether or not having benzene ring, possessing planarity, complete delocalisation of the electrons in the ring, following Huckel rule. Aromatic hydrocarbons are non- polar molecules and are usually colourless liquids or solids with a characteristic aroma. Arenes are characterised by electrophilic substitution reactions.
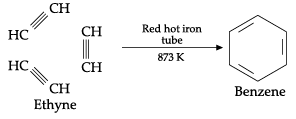
(i) Ethyne on passing through red hot iron tube at 873 K undergoes cyclic polymerisation. Name the product formed in this reaction.
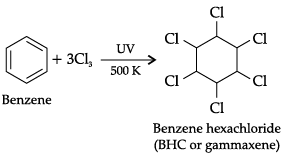
(ii) Write the chemical reaction for conversion of benzene into BHC.
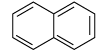
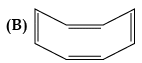
(iii) Examine the following and select the aromatic structures.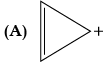
(iv) How does the presence of halogen atom affect the reactivity of benzene ring towards electrophilic substituition reaction?
OR
Write name and structure of any two arenes.
(i) In cyclic polymerisation, three molecules of ethyne polymerise to form benzene.
(ii)
(iii) In (A) part, ring is planar and follow (4n+2) π-electrons rule i.e., Huckel's rule. Here, n = 0.
(iv) For an electrophilic substitution reaction, the presence of halogen atom in the benzene ring deactivates the ring by inductive effect (–I effect) and increases the charge density at ortho- and para-position relative to meta-position by resonance. When chlorine is attached to benzene ring, chlorine being more electronegative pulls the electrons because of its -I effect. The electron cloud of benzene becomes less dense. Thus, chlorine makes the benzene ring in aryl halide somewhat deactivated. But due to resonance, the electron density on ortho- and para-position is greater than on meta-position.
OR
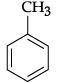
(i) Toluene
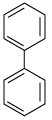
(ii) Biphenyl
(iii) Naphthalene
(or any other relevant arene and its structure)
FAQs on Class 11 Chemistry: CBSE Sample Question Papers- Term II (2021-22)- 1 - Sample Papers for Class 11 Medical and Non-Medical - JEE
| 1. What are the CBSE Sample Question Papers for Class 11 Chemistry Term II? |  |
| 2. How can I access the CBSE Sample Question Papers for Class 11 Chemistry Term II? |  |
| 3. Why should I solve the CBSE Sample Question Papers for Class 11 Chemistry Term II? |  |
| 4. How can I use the CBSE Sample Question Papers for Class 11 Chemistry Term II effectively? |  |
| 5. Are the CBSE Sample Question Papers for Class 11 Chemistry Term II similar to the actual exam papers? |  |