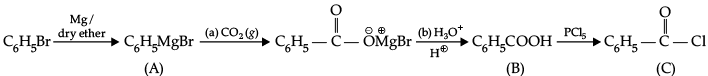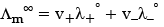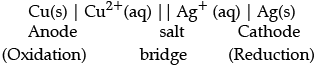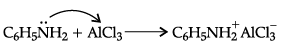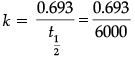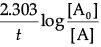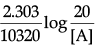Class 12 Chemistry: CBSE Sample Question Paper- Term II (2021-22)- 1 | Sample Papers for Class 12 Medical and Non-Medical PDF Download
| Table of contents |

|
| Class-XII |

|
| Time: 120 Minutes |

|
| Max. Marks: 35 |

|
| Section - A |

|
| Section - B |

|
| Section - C |

|
Class-XII
Time: 120 Minutes
Max. Marks: 35
General Instructions :
- There are 12 questions in this question paper with internal choice.
- SECTION A - Q. No. 1 to 3 are very short answer questions carrying 2 marks each.
- SECTION B - Q. No. 4 to 11 are short answer questions carrying 3 marks each.
- SECTION C- Q. No. 12 is case based question carrying 5 marks.
- All questions are compulsory.
- Use of log tables and calculators is not allowed
Section - A
Q.1. Give IUPAC name of (any 2):
(a) Succinic acid
(b) Oxalic acid
(c) Glutaric acid
(a) IUPAC name of succinic acid is Butane-1, 4-dioic acid.
(b) IUPAC name of oxalic acid is Ethane-1, 2-dioic acid.
(c) IUPAC name of glutaric acid is Pentane-1, 5-dioic acid.
Q.2. In a galvanic cell, the following cell reaction occurs:
Zn(s) + 2Ag+(aq) → Zn2+ (aq) + 2Ag(s); E°cell = +1.56V
(a) Is the direction of flow of electrons from zinc to silver or silver to zinc?
(b) How will concentration of Zn2+ ions and Ag+ ions be affected when the cell functions?
(a) Oxidation : Zn(s) → Zn+2(aq) + 2e–
Reduction : 2Ag+(eq) + 2e– → Ag(s)
Thus, electron flow from zinc to silver
(b) Concentration of Zn2+ ions will increase and Ag+ ions will decrease because zinc is being oxidised to zinc ion and silver ion is being reduced to silver.
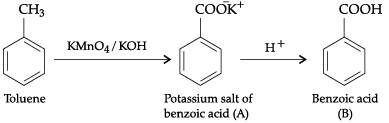
Q.3. Identify A and B and explain the given chemical reaction.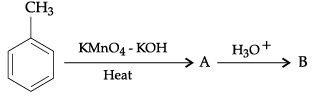
A is Potassium salt of benzoic acid and B is benzoic acid.
Section - B
Q.4. When a coordination compound CrCl3 .6H2O is mixed with AgNO3 solution, 3 moles of AgCl are precipitated per mole of the compound. Write:
(a) Structural formula of the complex.
(b) IUPAC name of the complex.
(c) Magnetic and spin behaviour of the complex.
OR
Write the hybridisation, shape and magnetic character of [Fe(CN)6]4−.
(a) [Cr(H2O)6]Cl3
(b) Hexaquachromium (III) chloride
(c) Paramagnetic and high spin
OR
Hybridisation: d2sp3
Shape: Octahedral
Magnetic character: Diamagnetic
Detailed Answer:Shape: Octahedral
There is no unpaired electron. Thus, it is diamagnetic in nature.
Q.5. (a) Which element of first transition series has highest 2nd ionization enthalpy ?
(b) Which element of first transition series has highest 3rd ionization enthalpy?
(c) Why Zn, Cd and Hg are not considered transition metals?
(a) Cu (Electronic configuration: [Ar]3d104s1) has the highest 2nd ionization enthalpy as the second electron to be removed from the completely filled d-orbital.
(b) Zinc (Electronic configuration [Ar]3d104s2) has the highest 3rd ionization enthalpy as third electron is to be removed from completely filled d-orbital and has maximum stability.
(c) Elements of Group 12, Zn Cd and Hg are not considered as transition metals due to their fully filled d-orbitals. These do not show general properties of transition elements.
Q.6. Write structures of compounds A, B and C in each of the following chemical reaction: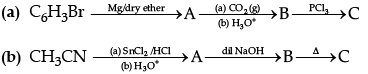
(a) A : C6H5MgBr
B : C6H5COOH
C : C6H5COCl
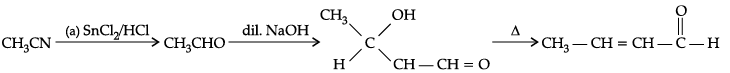
(b) A : CH3CHO
B : CH3CH(OH)CH2CHO
C : CH3CH = CHCHODetailed Answer :
(a)
(b)
Q.7. (a) What is a colloidal solution?
(b) Explain a dispersed phase and a dispersion medium with an example.
(c) What do you understand by adsorption isotherm?
(a) Colloidal solution is the heterogenous mixture in which a material (dispersed phase) is evenly suspended in a liquid (dispersion medium). Some of the examples of colloidal solution are gelatin, muddy water, butter, blood and coloured glass.
(b) Colloidal particles diameter ranges between 1 and 1000 nm.
The phase in which finely divided colloidal particles are dispersed is called the dispersed phase. The medium in which the colloidal particles are distributed is called the dispersion medium. Example: Starch represents the dispersed phase in a starch solution, while water is the dispersing medium.
(c) A graph drawn between of adsorption of gas on the adsorbent and the pressure of the gas at constant temperature is called adsorption isotherm.
Q.8: (a) Explain Kohlrausch's law of independent migration of ions.
(b) Depict the galvanic cell in which the given reaction takes place.
Cu(s) + 2Ag+(aq) → 2Ag(s) + Cu2+(aq)
(a) The Kohlrausch law of independent migration of ions states that limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte.
Limiting equivalent molar conductivity of an electrolyte is the algebraic sum of limiting molar equivalent conductivities of its constituent ions.
Where, v+, v– = Number of cations and anions respectively.Limiting molar conductivities of cations and anions respectively.
Limiting molar conductivity of electrolyte.
(b) Oxidation half reaction:
Cu(s) → Cu2+ (aq) + 2e−
Reduction half reaction:
Ag + (aq) + e− → Ag(s)
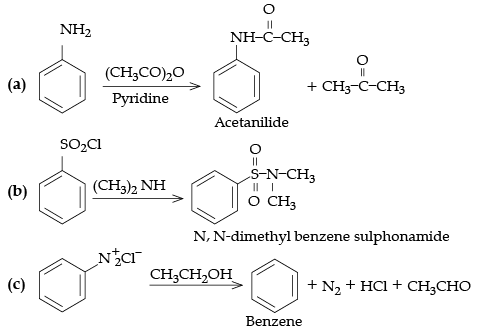
Q.9. Write the structures of the main products of the following reactions:
OR
(a) Give a simple chemical test to distinguish between aniline and N, N-dimethylaniline.
(b) Arrange the following in the increasing order of their pkb values: C6H5NH2, C2H5 NH2, C6H5NHCH3
(c) Write the IUPAC name of the compound given: (CH3CH2)2 NCH3.
(a) N–Phenylethanamide, C6H5NHCOCH3
(b) N, N-Dimethylbenzenesulphonamide, C6H5SO2N(CH3)2
(c) C6H6, BenzeneDetailed Answer:
OR
(a) Add chloroform in the presence of KOH and heat, then, aniline gives an offensive smell while N, N dimethylaniline does not. (or any other correct test)
(b) C2H5NH2< C6H5NHCH3< C6H5NH2
(c) N-Ethyl–N–methylethanamine.
Detailed Answer:
(a) Aniline being a primary amine and N, N-dimethyl aniline being a tertiary amine are distinguished by carbylamine test.
Primary aniline on heating with chloroform and ethanolic potassium hydroxide, a foul smell of isocyanides or carbylamines is observed. Aniline is aromatic primary amine, thus it gives positive test but N, N-dimethylaniline is tertiary amine, so it does not give carbylamine test.(b) Aliphatic amines are more basic than aromatic amines. Its is due to +I effect of alkyl groups of aliphatic amines and –R effect of aromatic amines. Thus, order of basic strength is:
C6H5NH2 < C6H2NHCH3 < C2H5NH2
Hence, order of pkb is:
C2H5NH2 < C6H5NHCH3 < C6H5NH2
Q.10. Give reasons:
(a) Aniline does not undergo Friedal Crafts reaction.
(b) Aromatic primary amines cannot be prepared by Gabriel’s phthalimide synthesis.
(c) Aliphatic amines are stronger base than ammonia.
OR
How does the following conversions take place as given below:
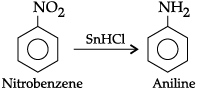
(a) Nitrobenzene into aniline
(b) Ethanoic acid into methanamine
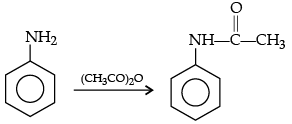
(c) Aniline to N-phenylethanamide
(Write the chemical equations involved.)
(a) Aniline does not undergo Friedel-Crafts reaction because aniline being a lewis base forms a salt with AlCl3 which is a lewis acid. The amino group is not in a position to activate the benzene ring towards electrophilic substitution. Therefore, the reaction is not possible.
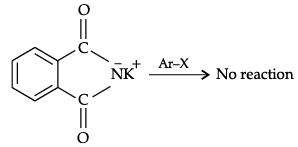
(b) Aromatic primary amines can not be prepared by Gabriel’s phthalimide synthesis because aryl halides do not undergo nucleophilic substitutions (SN2) with the potassium salt of phthalimide. So, the bond cleavage does not take place.
(c) Aliphatic amines are stronger base than ammonia because the alkyl group in aliphatic amines has +I effect. So, the alkyl group tends to increase the electron density on the nitrogen atom. Thus, the electron releasing tendency of amines becomes more than that of ammonia.
OR
(a) Nitrobenzene into aniline(b) Ethanoic acid into methanamine
(c) Aniline to N-Phenylethanamide
Q.11. (a) Although, Zr belongs to 4d and Hf belongs to 5d transition series but it is quite difficult to separate them, explain why?
(b) Out of Cu+ and Cu2+, which ion is unstable in aqueous solution and why?
OR
Give reasons:
(a) Transition metals and their compounds show catalytic activities.
(b) Separation of a mixture of Lanthanoid elements is difficult.
(c) Zn, Cd and Hg are soft and have low melting point.
(a) Zr, a member of 4d series and Hf, a member of 5d series, belong to the same group (Group 4). They are chemically so similar that their separation is difficult. This is because both have almost similar size due to lanthanide contraction. The radii of these elements are 160 pm (Zr) and 159 pm (Hf).
(b) Cu+, due to disproportionation reaction and low hydration enthalpy.
OR
(a) The catalytic activities of transition metals and their compounds is due to the ability of adopt variable oxidation states and to form complexes. It can also provide a large surface area for the reactants to be adsorbed.
(b) Separation of lanthanoid elements is difficult because all lanthanoid elements have almost similar physical as well as chemical properties. Due to the lanthanoid contraction the change in the atomic or ionic radii is very small.
(c) Zn, Cd and Hg are soft and have low melting point because no d-orbitals are available for metallic bond formation and bonds formed are very weak.
Section - C
Q.12. Read the passage given below and answer the questions that follow.
Are there nuclear reactions going on in our bodies?
There are nuclear reactions constantly occurring in our bodies, but there are very few of them compared to the chemical reactions, and they do not affect our bodies much. All of the physical processes that take place to keep a human body running are chemical processes. Nuclear reactions can lead to chemical damage, which the body may notice and try to fix. The nuclear reaction occurring in our bodies is radioactive decay. This is the change of a less stable nucleus to a more stable nucleus. Every atom has either a stable nucleus or an unstable nucleus, depending on how big it is and on the ratio of protons to neutrons. The ratio of neutrons to protons in a stable nucleus is thus around 1:1 for small nuclei (Z < 20). Nuclei with too many neutrons, too few neutrons, or that are simply too big are unstable. They eventually transform to a stable form through radioactive decay. Wherever there are atoms with unstable nuclei (radioactive atoms), there are nuclear reactions occurring naturally. The interesting thing is that there are small amounts of radioactive atoms everywhere: in your chair, in the ground, in the food you eat, and yes, in your body.
The most common natural radioactive isotopes in humans are carbon-14 and potassium-40. Chemically, these isotopes behave exactly like stable carbon and potassium. For this reason, the body uses carbon-14 and potassium-40 just like it does normal carbon and potassium; building them into the different parts of the cells, without knowing that they are radioactive. In time, carbon-14 atoms decay to stable nitrogen atoms and potassium-40 atoms decay to stable calcium atoms. Chemicals in the body that relied on having a carbon-14 atom or potassium-40 atom in a certain spot will suddenly have a nitrogen or calcium atom. Such a change damages the chemical. Normally, such changes are so rare, that the body can repair the damage or filter away the damaged chemicals.
The natural occurrence of carbon-14 decay in the body is the core principle behind carbon dating. As long as a person is alive and still eating, every carbon-14 atom that decays into a nitrogen atom is replaced on average with a new carbon-14 atom. But once a person dies, he stops replacing the decaying carbon-14 atoms. Slowly the carbon-14 atoms decay to nitrogen without being replaced, so that there is less and less carbon-14 in a dead body. The rate at which carbon-14 decays is constant and follows first order kinetics. It has a half - life of nearly 6000 years, so by measuring the relative amount of carbon-14 in a bone, archeologists can calculate when the person died. All living organisms consume carbon, so carbon dating can be used to date any living organism, and any object made from a living organism. Bones, wood, leather, and even paper can be accurately dated, as long as they first existed within the last 60,000 years. This is all because of the fact that nuclear reactions naturally occur in living organisms.
(a) Why is Carbon -14 radioactive while Carbon -12 not? (Atomic number of Carbon: 6)
(b) Researchers have uncovered the youngest known dinosaur bone, dating around 65 million years ago. How was the age of this fossil estimated?
(c) Which are the two most common radioactive decays happening in human body?
(d) Suppose an organism has 20 g of Carbon -14 at its time of death. Approximately how much Carbon -14 remains after 10,320 years? (Given antilog 0.517 = 3.289)
OR
(d) Approximately how old is a fossil with 12 g of Carbon -14 if it initially possessed 32 g of Carbon -14? (Given log 2.667 = 0.4260)
(a) Ratio of neutrons to protons is 1.3:1 which is not the stable ratio of 1:1
(b) Age of fossils can be estimated by C-14 decay. All living organisms have C-14 which decays without being replaced back once the organism dies.
(c) Carbon-14 atoms decay to stable nitrogen atoms and potassium-40 atoms decay to stable calcium.
(d) t = 2.303/ k log (Co/Ct)
Co = 20 g, Ct = ?
t = 10320 years; k = 0.693/6000 (half-life given in passage)
Substituting in equation:
10320 = 2.303 / (0.693/6000) log 20/ Ct
0.517 = log 20 / Ct anlilog (0.517) = 20/Ct
3.289 = 20/Ct
Ct = 6.17 g
Detailed Answer:
[Ao] = 20g; [A] = ?
t = 10,320 years
t1/2 = 6000 years (given in passage)
So,
= 0.0001155 years–1
Now, k =
0.0001155=
0.517 = log (20/[A])
antilog (0.517) = (20/[A])
3.289 = 20/[A]
[A] = 6.08g
So, 6.08g of carbon-14 remains after 10,320 years.
OR
t = 2.303/ k log (Co/Ct)
Co = 32 g, Ct = 12
t = ?, k = 0.693/6000 (half life given in passage)
substituting in equation:
t = 2.303/(0.693/6000) log 32/12
t = 2.303 × 60000/0.693 log 2.667
t = 2.303 × 6000 × 0.4260/0.693
= 8494 years
|
159 docs|4 tests
|
FAQs on Class 12 Chemistry: CBSE Sample Question Paper- Term II (2021-22)- 1 - Sample Papers for Class 12 Medical and Non-Medical
| 1. What is the duration of Class XII Chemistry exam? |  |
| 2. What is the maximum marks for the Class XII Chemistry exam? |  |
| 3. How many sections are there in the Class XII Chemistry exam? |  |
| 4. What is the difficulty level of the questions in Section A? |  |
| 5. What is the format of the CBSE Sample Question Paper for Class XII Chemistry? |  |