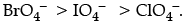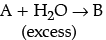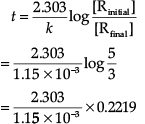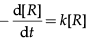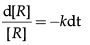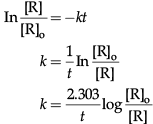Class 12 Chemistry: CBSE Sample Question Paper- Term II (2021-22)- 2 | Sample Papers for Class 12 Medical and Non-Medical PDF Download
| Table of contents |

|
| Class-XII |

|
| Time: 120 Minutes |

|
| Max. Marks: 35 |

|
| Section - A |

|
| Section - B |

|
| Section - C |

|
Class-XII
Time: 120 Minutes
Max. Marks: 35
General Instructions :
- There are 12 questions in this question paper with internal choice.
- SECTION A - Q. No. 1 to 3 are very short answer questions carrying 2 marks each.
- SECTION B - Q. No. 4 to 11 are short answer questions carrying 3 marks each.
- SECTION C- Q. No. 12 is case based question carrying 5 marks.
- All questions are compulsory.
- Use of log tables and calculators is not allowed
Section - A
Q.1. Explain redox potential. Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.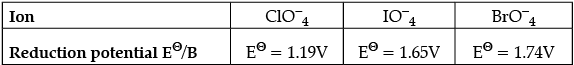
Redox potential (also known as reduction/ oxidation potential) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is measured in volts (V), or millivolts (mV). The more positive the reduction potential of a species, the greater the species’ affinity for the electrons and tendency to be reduced.
The higher the reduction potential, the higher is its tendency to get reduced. Hence, the order of oxidising power is:
Q.2. (a) What is the order of the reaction whose rate constant has same units as the rate of reaction ?
(b) For a reaction A + H2O → B; Rate ∝ [A]. What is the order of this reaction?
(a) Zero order
(b) Pseudo-first order
Detailed Answer:
(a) Rate of reaction, R = k[A]n
Where, n = Order of the reaction
k = Rate constant
If unit of [R] = [k]
Then, n = 0 (zero order reaction)
(b) Given,
Rate of reaction, R = k[A][H2O]
But, if H2O is in excess, it will not be affecting rate of reaction and remain constant such that,
R = k'[A]
Thus, R ∝ [A]
It is considered as pseudo first order reaction.
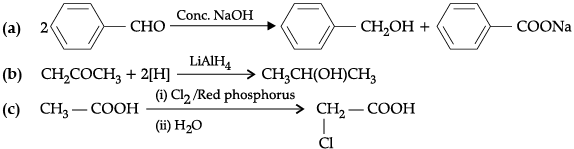
Q.3. Complete the following chemical equations: (any two)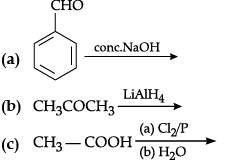
Section - B
Q.4. (a) Why is Cr+2 is reducing agent and Mn+3 is oxidizing agent while both have 3d4 configuration?
(b) Write the electron configuration of elements with atomic numbers 91 and 109.
(a) E° value for Cr3+/Cr2+ is negative (–0.41 V) whereas, E° values for Mn3+/ Mn2+ is positive (+1.57 V). Hence, Cr2+ ion can easily undergo oxidation to give Cr3+ ion and, therefore, act as strong reducing agent whereas, Mn3+ can easily undergo reduction to give Mn2+ and hence act as an oxidizing agent.
(b)
Q.5. The following curve is obtained when molar conductivity (∧m) is plotted against the square root of concentration, C1/2 for two electrolytes A and B: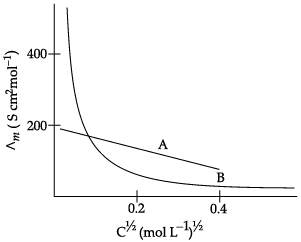
(a) How do you account for the increase in the molar conductivity of the electrolyte A on dilution?
(b) As seen from the graph, the value of limiting molar conductivity (∧°m) for electrolyte B cannot be obtained graphically. How can this value be obtained?
(c) Define limiting molar conductivity.
(a) As seen from the graph, electrolyte A is a strong electrolyte which is completely ionised in solution. With dilution, the ions are far apart from each other and hence the molar conductivity increases.
(b) To determine the value of limiting molar conductivity for electrolyte B, indirect method based upon Kohlrausch’s law of independent migration of ions is used.
(c) When concentration approaches zero, the molar conductivity is known as limiting molar conductivity.
Q.6. (a) Write any two characteristics of chemisorption.
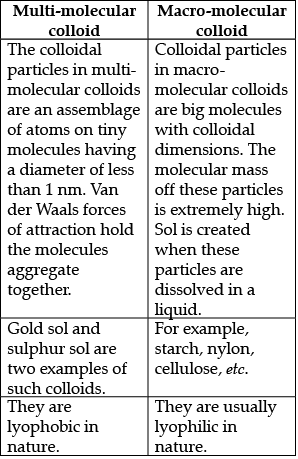
(b) Explain multi-molecular and macro-molecular colloids.
(a) (i) Chemisorption is a specific process that only occurs if there is a chemical bond between adsorbent and adsorbate.
(ii) Chemisorption is an endothermic reaction, so it is inversely proportional to temperature.
(b)
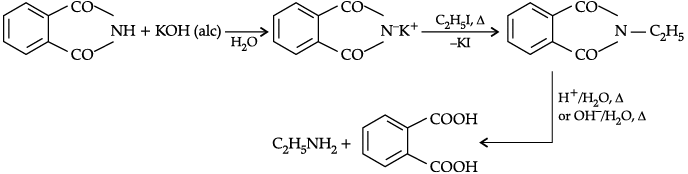
Q.7. (a) Explain Gabriel Phthalimide synthesis with example.
(b) Give the IUPAC names of following:
(i) m-BrC6H4NH2
(ii) CH3NHCH (CH3)2
OR
(a) How will you convert benzanamide to toluene?
(b) Ketones are less reactive than aldehydes. Why?
(a) Gabriel phthalimide synthesis: It is a method of preparation of pure aliphatic and aralkyl primary amines. Phthalimide on treatment with ethanolic KOH gives potassium phathalimide which on heating with a suitable alkyl or aralkyl halides gives N-substituted phthalimides, which on hydrolysis with dil. HCl or with alkali give primary amines.
(b) (i) 3-Bromobenzenamine
or 3-bromoaniline
(ii) N-Methylpropan-2-amine
OR
(a)
(b) Ketones are less reactive than aldehydes due to following reasons:
(i) Electron releasing effect
In ketones, the carbonyl carbon is attached to alkyl groups which are electron releasing in nature. These alkyl groups push electrons towards carbonyl carbon and therefore, decrease the magnitude of positive charge on it and make it less reactive towards nucleophilic attack.
(ii) Steric effect
In ketones, the presence of two bulky alkyl groups also hinders the approach of the nucleophile to attack the carbonyl carbon.
Q.8. (a) (i) Transition metals form alloys. Why?
(ii) Chromium is typically hard metal but mercury is liquid. Why?
(b) Based on the data, arrange Fe2+, Mn2+ and Cr2+ in the increasing order of stability of +2 oxidation state.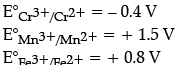
OR
Give reasons:
(a) E° value for Mn3+/Mn2+ is much more positive than that for Fe3+/Fe2+.
(b) Iron has higher enthalpy of atomisation than that of copper.
(c) Sc3+ is colourless in aqueous solution whereas Ti3+ is coloured.
(a) (i) Due to the almost similar atomic sizes of elements, atoms can easily occupy the position in the crystal lattice of the other atom of another element (metal). Thus, easily formation of alloys is the property of transition metals.
(ii) Due to the presence of large number of unpaired electrons, metal-metal interaction is strong whereas mercury does not have unpaired electrons and forms weak metallic bonds.
(b) Higher the reduction potential, higher will be the ability to reduce itself to attain +2 oxidation state.
Thus, order of stability of +2 oxidation state.
Cr2+ < Fe2+ < Mn2+
OR
(a) Because Mn2+ is more stable than Mn3+ due to half-filled d5 configuration whereas Fe2+ becomes unstable after gaining an electron to its half-filled orbital.
(b) Due to presence of higher number of unpaired electrons in iron, they have stronger metallic bonding. Hence, the enthalpy of atomisation is more of iron than that of copper.
(c) Sc3+ (3d°) is colourless as it does not contain unpaired electrons to undergo d-d transition while Ti3+(3d1) is coloured as it contains unpaired electrons to undergo d-d transition by absorbing light from visible region and radiate complementary colour.
Q.9. Following ions are given: Cr2+, Cu2+, Cu+, Fe+2, Fe3+, Mn+3
Identify the ion which is
(a) A strong reducing agent
(b) Unstable in aqueous solutions
(c) A strong oxidizing agent.
Give reasons to support your answer.
OR
(a) What is the IUPAC name of the complex [Ni(NH3)6]Cl2?
(b) What is meant by chelate effect?
(c) Which of the following is more stable complex and why?
[Co(NH3)6]3+ and [Co(en)3]3+.
(a) Cr2+ is strong reducing agent because its electronic configuration changes from d4 to d5 and have half-filled t2g level.
(b) Cu+ in aqueous medium is required to remove one electron from Cu+ to Cu+2, high hydration energy of Cu+2 compensates for it. Therefore, Cu+ ion in an aqueous solution is unstable.
2Cu+ → Cu+2(eq) + Cu(s)
(c) Mn3+ is strong oxidising agent because configuration changes from d4 to d5 when Mn3+ is reduced to Mn+2, and d5 (half-filled) configuration is extra stable.
OR
(a) Hexaamminenickel (II) Chloride.
(b) Formation of stable complex with a polydentate ligand due to stronger bonding is known as chelate effect.
(c) [Co(en)3]3+: Because (en) is a chelating ligand/bidentate ligand.
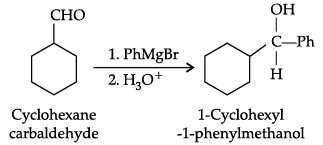
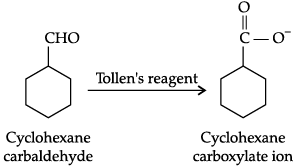
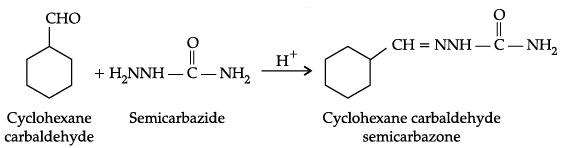
Q.10. Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
(a) PhMgBr and then H3O+
(b) Tollens' reagent
(c) Semicarbazide in weak acidic medium.
(a)
(b)
(c)
Q.11. (a) Give a chemical test to distinguish between N-methylethanamine and N,N-dimethyl ethanamine.
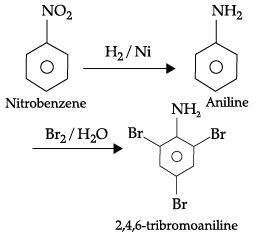
(b) Write the reaction for catalytic reduction of nitrobenzene followed by reaction of product so formed with bromine water.
(c) Out of butan-1-ol and butan-1-amine, which will be more soluble in water and why?
OR
Arrange the following in increasing order of specified property:
(a) Aniline, ethanamine, 2-ethylethanamine (solubility in water)
(b) Ethanoic acid, ethanamine, ethanol (boiling point)
(c) Methanamine, N, N- dimethylmethanamine and N- methylmethanamine (basic strength in aqueous phase)
(a) N-methylethanamine is a secondary amine. When it reacts with benzene sulphonyl chloride, it forms N- Ethyl -N methyl sulphonamide, where as N, N-dimethyl ethanamine so, is a tertiary amine so, it does not react with benzenesulphonyl chloride.
(b)(c) Butan-1-ol
Alcohol forms stronger hydrogen bonds with water than formed by amine due to higher electronegativity of oxygen in alcohol than nitrogen in amine.
OR
(i) Aniline < N-ethylethanamine < Ethanamine
Ethanamine form intermolecular H-bonds with water. N-ethyl ethanamine has bulky alkyl group that form weak H-bonds. But aniline does not form H-bonds due to hydrophobic – C6H5 group attached to amine.
(ii) Ethanamine < ethanol < ethanoic acid
In liquid state, two ethanoic acid molecules are held together by two H-bonds to form dimer. Thus, bigger size of dimer has great Van Der Waals forces. So, ethanoic acid has higher boiling point than ethanol. Also, alcohols have higher boiling point than amines.
(iii) N, N dimethylmethanamine < methanamine < N-methylmethanamine
Order of basic strength of amines in aqueous solution depends upon +I effect of alkyl groups, solution effect and steric hindrance. Greater the size of ion, lesser is the solution.
Section - C
Q.12. Read the passage given below and answer the following questions:
Concentration dependence of rate is called differential rate equation. Integrated differential equations give relation between directly measured experimental data i.e., concentration at different times and rate constant. The integrated rate equations are different for the reactions of different reaction orders. The first-order reaction has a rate constant 1.15 × 10–3 s–1.
(a) How long will 5 g of this reactant take to reduce to 3 g?
(b) Under which condition a bimolecular reaction is kinetically first order reaction?
(c) Calculate the half life of the reaction.
(d) Derive integrated rate equation for rate constant of a first order reaction.
OR
(d) For a chemical reaction R → P, variation in ln [R] vs time (t) plot is given below: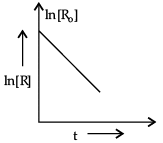
For this chemical reaction:
(i) Predict the order of reaction.
(ii) What is the unit of rate constant (k)?
(a) Initial amount = 5 g
Final concentration = 3g
Rate constant= 1.15 × 10–3 s–1
For a first order reaction
= 444.379s
(b) For a reaction, A + B → P
Rate = k[A][B]
If B is in excess then, R = k’[A].
Thus, a bimolecular reaction is kinetically first order reaction, if concentration of due of the reaction is in excess such that it does not affect the rate of reaction.
(c) Given,
k = 1.15 × 10–3 s–1
For first order reaction,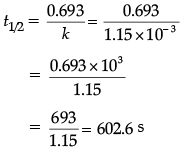
(d) R → P
Rate =
or
Integrating this equation, we get
ln [R] = –kt + I …(i)
When t = 0, R = [R]o,
where [R]o is the initial concentration of the reactant.
Therefore, equation (i) can be written as
In [R]o = –k × 0 + I
In [R]o = I
Substituting the value of I in equation (i)
In [R] = –kt + ln[R]o
Rearranging this equation
(d) (i) First order.
(ii) s–1/time–1
Detailed Answer:
(d) (i) The given graph is of first order reaction where slope is (– k)
For first order reaction, ln[R] = –kt + ln[Ro]
compare with y = mx + c
Slope = –k
(ii) [kt] is dimensionless number unit of k = [t–1] = s–1.
|
159 docs|4 tests
|