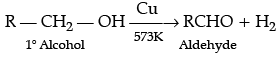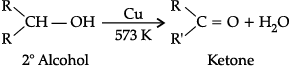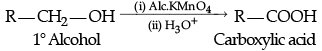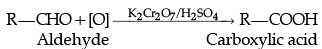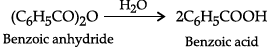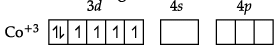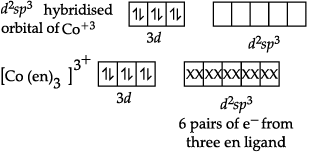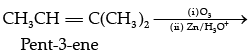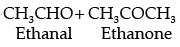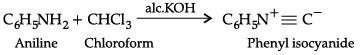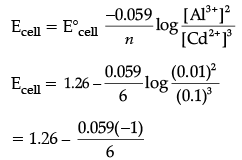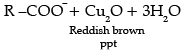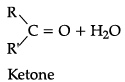Class 12 Chemistry: CBSE Sample Question Paper- Term II (2021-22)- 5 | Sample Papers for Class 12 Medical and Non-Medical PDF Download
| Table of contents |

|
| Class-XII |

|
| Time: 120 Minutes |

|
| Max. Marks: 35 |

|
| Section - A |

|
| Section - B |

|
| Section - C |

|
Class-XII
Time: 120 Minutes
Max. Marks: 35
General Instructions :
- There are 12 questions in this question paper with internal choice.
- SECTION A - Q. No. 1 to 3 are very short answer questions carrying 2 marks each.
- SECTION B - Q. No. 4 to 11 are short answer questions carrying 3 marks each.
- SECTION C- Q. No. 12 is case based question carrying 5 marks.
- All questions are compulsory.
- Use of log tables and calculators is not allowed
Section - A
Q.1. Explain preparation of the following (any 2):
(a) A ldehyde by dehydrogenation of alcohol.
(b) K etones by dehydrogenation of alcohol.
(c) Carboxylic acids by oxidation of primary alcohols.
(a) Preparation of aldehyde by dehydrogenation of alcohols:
(b) Preparation of ketone by dehydrogenation of alcohols
(c) By oxidation of primary alcohols and aldehydes:
Q.2. Following reactions can occur at cathode during the electrolysis of aqueous silver nitrate solution using Pt electrodes:
Ag+ (aq) + e– → Ag (s); E° = 0.80 V
H+ (aq) + e– → 1/2 H2 (g); E° = 0.00 V.
On the basis of their standard electrode potential values, which reaction is feasible at cathode and why?
The reaction Ag+(aq) + e– → Ag(s) will be feasible at cathode because it has higher reduction potential.
As reaction with higher value of standard electrode potential occurs at cathode, Ag gets reduced. So, the reaction occurring at cathode
Ag+ (aq) + e– → Ag(s)
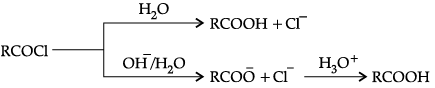
Q.3. Explain the preparation of carboxylic acid from acyl halides and anhydrides.
When acid chloride is hydrolysed with water then carboxylic acid is produced and it is more readily hydrolysed with aqueous base and gives corresponding carboxylic ions which on further acidification gives the carboxylic acid.
Anhydrides are hydrolysed with water to form carboxylic acid.
Section - B
Q.4: Account for the following:
(a) Aniline cannot be prepared by the ammonolysis of chlorobenzene under normal conditions.
(b) N-ethylethanamine boils at 329.3K and butanamine boils at 350.8K, although both are isomeric in nature.
(c) Acylation of aniline is carried out in the presence of pyridine.
OR
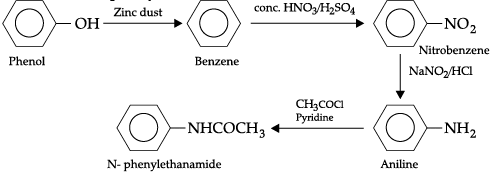
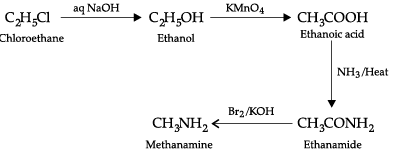
Convert the following:
(a) Phenol to N-phenylethanamide.
(b) Chloroethane to methanamine.
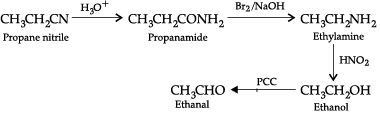
(c) Propanenitrile to ethanal.
(a) In case of chlorobenzene, the C—Cl bond is quite difficult to break as it acquires a partial double bond character due to conjugation. So, under the normal conditions, ammonolysis of chlorobenzene does not yield aniline.
(b) Primary and secondary amines are engaged in intermolecular association due to hydrogen bonding between nitrogen of one molecule and hydrogen of another molecule. Due to the presence of three hydrogen atoms, the intermolecular association is more in primary amines than in secondary amines as there are two hydrogen atoms available for hydrogen bond formation in it.
(c) During the acylation of aniline, stronger base pyridine is added. This is done in order to remove the HCl so formed during the reaction and to shift the equilibrium to the right hand side.OR
(a) Phenol into N-phenylethanamide(b) Chloroethane to methanamine
(c) Propane nitrile to ethanal
Q.5. Answer the following questions:
(a) [Ni(H2O)6] 2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+ (aq) is blue in colour, give reason in support of your answer .
(b) Write the formula and hybridization of the following compound: tris(ethane-1,2–diamine) cobalt(III) sulphate
OR
In a coordination entity, the electronic configuration of the central metal ion is 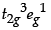
(a) Is the coordination compound a high spin or low spin complex?
(b) Draw the crystal field splitting diagram for the above complex.
(a) The colour of coordination compound depends upon the type of ligand and d-d transition taking place.
H2O is weak field ligand , which causes small splitting , leading to the d-d transition corresponding green colour, however due to the presence of (en) which is a strong field ligand , the splitting is increased. Due to the change in t2g eg splitting, the colouration of the compound changes from green to blue.
(b) Formula of the compound is [Co(H2NCH2CH2NH2)3]2 (SO4)3
Hybridisation of the compound: d2sp3.
Detailed Answer:
(b) [Co(H2NCH2CH2NH2)3]2 (SO4)3
Here, en is a neutral ligand.
Oxidation state of Co is +3.
Electronic configuration of Co = [Ar]3d74s2
Electronic configuration of Co+3 = [Ar]3d6
en is strong field ligand so back pairing of electrons will occur.So, hybridization of [Co(en)3]2(SO4)3 is d2sp3
OR
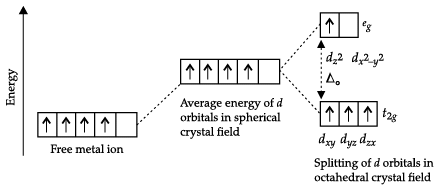
(a) As the fourth electron enters one of the eg orbitals giving the configuration t2g3 eg1, which indicates Δo < P hence, forms high spin complex.
(b)
Q.6. Account for the following:
(a) Ti(IV) is more stable than Ti (II) or Ti(III).
(b) In case of transition elements, ions of the same charge in a given series show progressive decrease in radius with increasing atomic number.
(c) Zinc is comparatively a soft metal, iron and chromium are typically hard.
(a) Ti is having electronic configuration [Ar] 3d2 4s2. Ti (IV) is more stable as Ti4+ acquires nearest noble gas configuration on loss of 4 e-.
(b) In case of transition elements, ions of the same charge in a given series show progressive decrease in radius with increasing atomic number.
As the new electron enters a d orbital each time, the nuclear charge increases by unity. The shielding effect of a d electron is not that effective, hence the net electrostatic attraction between the nuclear charge and the outermost electron increases and the ionic radius decreases.
(c) Iron and Chromium are having high enthalpy of atomization due to the presence of unpaired electrons, which accounts for their hardness. However, zinc has low enthalpy of atomization as it has no unpaired electron. Hence, zinc is comparatively a soft metal.
Q.7. An alkene ‘A’ (Mol. formula C5H10) on ozonolysis gives a mixture of two compounds ‘B’ and ‘C ’. Compound ‘B’ gives positive Fehling’s test and also forms iodoform on treatment with I2 and NaOH. Compound ‘C’ does not give Fehling’s test but forms iodoform. Identify the compounds A, B and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
Compound A is an alkene, on ozonolysis it will give carbonyl compounds. As both B and C have,
group, B gives positive Fehling’s test so it is an aldehyde and it gives iodoform test so, it has CH3C = O group. This means that aldehyde is acetaldehyde.
C does not give Fehling’s test, so it is a ketone. It gives positive iodoform test so it is a methyl ketone means it has CH3C = O group.
Compound A (C5H10) on ozonlysis gives B (CH3CHO) + C (CH3COR). So, “C” is CH3COCH3.
CH3CHO + 2Cu2+ + 5OH– → CH3COO– + Cu2O (red ppt) + 3H2O
CH3COCH3 + 2Cu2+ + 5OH– → No reaction
CH3CHO + 3I2 + 3 NaOH → CHI3 (yellow ppt) + 3HI + HCOONa
CH3COCH3 + 3I2 + 3NaOH → CHI3 (yellow ppt) + 3HI + CH3COONa
A = CH3CH=C(CH3)2
B = CH3CHO
C = CH3COCH3
Q.8. Observe the figure given below and answer the questions that follow: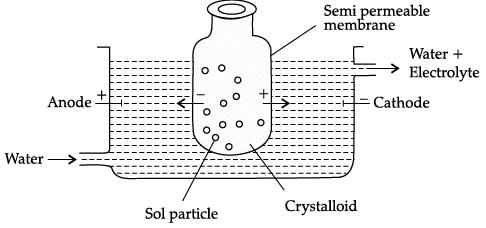
(a) Which process is represented in the figure?
(b) What is the application of this process?
(c) Can the same process occur without applying electric field? Why is the electric field applied?
(a) Electrodialysis
(b) Purification of colloidal solution
(c) Yes, dialysis is a very slow process, To increase speed of dialysis, electric field is applied.
Q.9. What happens when reactions:
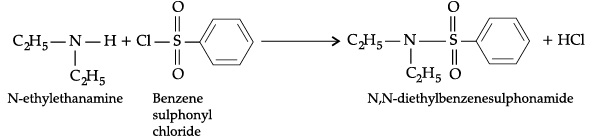
(a) N-ethylethanamine reacts with benzenesulphonyl chloride.
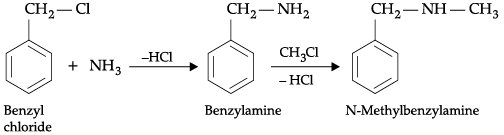
(b) Benzylchloride is treated with ammonia followed by the reaction with Chloromethane.
(c) Aniline reacts with chloroform in the presence of alcoholic potassium hydroxide.
OR
(a) Write the IUPAC name for the following organic compound: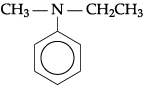
(b) Complete the following: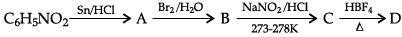
(a) When N-ethylethanamine reacts with benzenesulphonyl chloride, N, N-diethylbenzenesulphonamide is formed.
(b) When benzylchloride is treated with ammonia, benzylamine is formed which on reation with chloromethane yields a secondary amine, N-methylbenzylamine.
(c) When aniline reacts with chloroform in the presence of alcoholic potassium hydroxide, phenyl isocyanides or phenyl isonitrile is formed.
Detailed Answer:
(a)
(b)
(c)
OR
(a) N-Ethyl-N-methylbenzenamine or N-Ethyl-N-ethylaniline
(b)
Q.10. Represent the cell in which the following reaction takes place. The value of E˚ for the cell is 1.260 V. What is the value of Ecell?
2Al(s) + 3Cd2+ (0.1M) → 3Cd (s) + 2Al3+ (0.01M)
Al(s)|Cd2+ (0.1M) || Al3+ (0.01M)|Cd(s) 2Al(s) + 3Cd2+ (0.1M) → 3Cd (s) + 2Al3+ (0.01M)
= 1.26 + 0.009
= 1.269 V
Q.11. (a) Why are fluorides of transition metals more stable in their higher oxidation state as compared to the lower oxidation state?
(b) Which one of the following would feel attraction when placed in magnetic field: Co2+, Ag+, Ti4+, Zn2+?
(c) It has been observed that first ionization energy of 5d series of transition elements are higher than that of 3d and 4d series, explain why?
OR
On the basis of the figure given below, answer the following questions: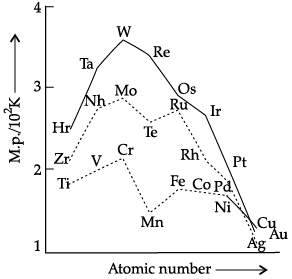 (a) Why manganese has lower melting point than chromium?
(a) Why manganese has lower melting point than chromium?
(b) Why do transition metals of 3d series have lower melting points as compared to 4d series?
(c) In the third transition series, identify and name the metal with the highest melting point.
(a) The ability of fluorine to stabilize the highest oxidation state is attributed to the higher lattice energy or high bond enthalpy.
(b) Co2+ has three unpaired electrons so, it would be paramagnetic in nature, hence Co2+ ion would be attracted to magnetic field.
(c) The transition elements of 5d series have intervening 4f orbitals. There is greater effective nuclear charge acting on outer valence electrons due to the weak shielding by 4f electrons. Hence, first ionisation energy of 5d series of transition elements are higher than that of 3d and 4d series.
OR
(a) With increase in unpaired electrons, melting point increases. In Mn (3d24s2), pairing starts after Cr(3d54s1). Thus, there is weak metallic bonding further due to less unpaired electrons. It reduces enthalpy of atomization value. Hence, manganese has lower melting point than chromium.
(b) There is much more frequent metal – metal bonding in compounds of the heavy transition metals i.e., 4d and 5d series, which accounts for lower melting point of 3d series.
(c) Tungsten
Section - C
Q.12. Read the passage given below and answer the questions that follow.
Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl group. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols or hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by the treatment of Grignard reagents with carbon dioxide.
(a) Name a method by which both aldehydes and ketones can be prepared.
(b) How will you distinguish between aliphatic aldehydes and aromatic aldehydes ?
(c) How ketones are prepared by the oxidation of secondary alcohol?
(d) Name the compounds A and B formed in the following reaction: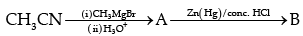
OR
Give a method through which number of carbon atoms can be increased in a chain.
(a) Both aldehydes and ketones can be prepared by oxidation of alcohols.
(b) On heating an aldehyde with Fehling’s reagent, a reddish brown precipitate is obtained. Aldehydes are oxidised to corresponding carboxylate anion. Aromatic aldehydes do not respond to this test.
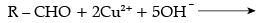
(c)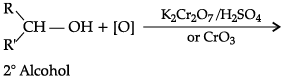
(d)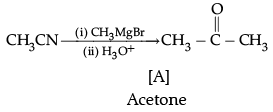
OR
The number of C-atoms can be increased in the chain by Grignard reaction.
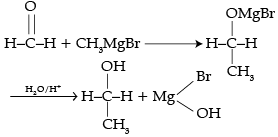
|
159 docs|4 tests
|