Class 7 Science Chapter 2 NCERT Based Activity - Exploring Substances: Acidic, Basic and Neutral
Activity 2.1: Let us explore
- Collect samples of lemon juice, soap solution, amla juice, tamarind water, vinegar, baking soda solution, lime water, tap water, washing powder solution, sugar solution, and salt solution.
- Take a strip of blue litmus paper and cut it into small pieces.
- Spread these pieces on a clean and dry white tile.
- Using a dropper, put one drop of each of the samples, one-by-one, on these litmus paper pieces, as shown in Fig. 2.2a.
 Fig. 2.2(a): Colour change in blue litmus paper
Fig. 2.2(a): Colour change in blue litmus paper
- Do you observe any change in the colour of the blue litmus pieces?
- Record your observations in Table 2.1.
- Repeat the same activity with pieces of red litmus paper as shown in Fig. 2.2b and record your observations in Table 2.1.
 Fig. 2.2(b): Colour change in red litmus paper
Fig. 2.2(b): Colour change in red litmus paper - Testing the nature of samples with blue and red litmus papers
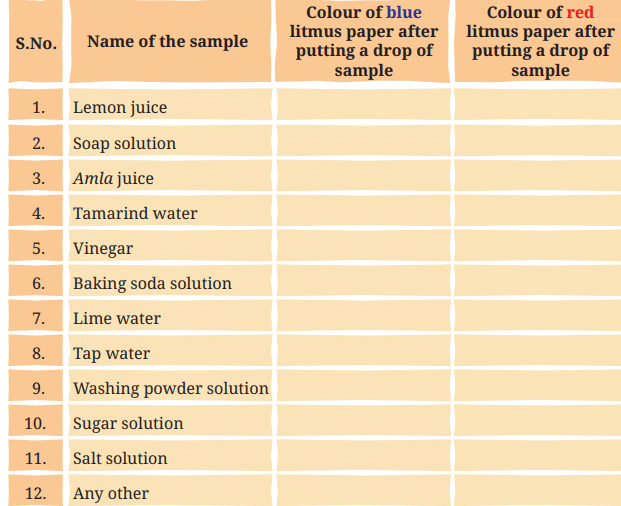 Table 2.1Do not confuse lime water with the word lime, which is a fruit similar to lemon. Lime water (solution of calcium hydroxide in water) can be easily prepared by mixing lime (chuna, i.e. calcium oxide) in water and leaving it undisturbed for some time, say an hour. Filter the liquid into another container and use it as lime water.
Table 2.1Do not confuse lime water with the word lime, which is a fruit similar to lemon. Lime water (solution of calcium hydroxide in water) can be easily prepared by mixing lime (chuna, i.e. calcium oxide) in water and leaving it undisturbed for some time, say an hour. Filter the liquid into another container and use it as lime water.
Ans: Based on the document, substances are classified as acidic (turn blue litmus to red), basic (turn red litmus to blue), or neutral (no change in either). Using this, Let's complete Table 2.1 for the samples listed.
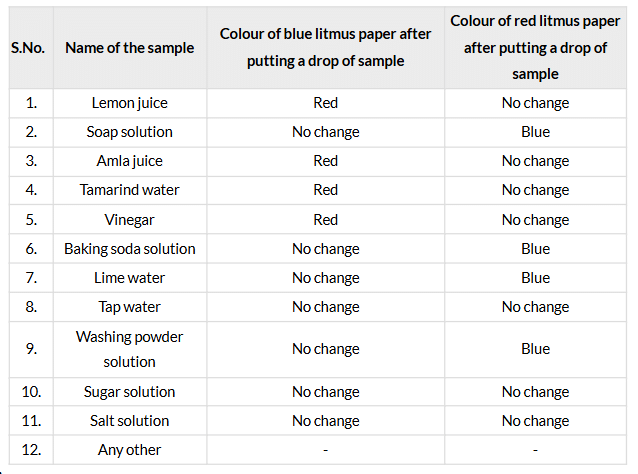
Now, let us analyse Table 2.1 and sort the samples into three groups as follows —
- Group A with samples that turn the blue litmus paper to red.
- Group B with samples that turn the red litmus paper to blue.
- Group C with samples that do not affect either of the two litmus papers. Record the data in Table 2.2.
 Table 2.2Table 2.2: Grouping of samples tested in Table 2.1
Table 2.2Table 2.2: Grouping of samples tested in Table 2.1
Ans: Based on Table 2.1.
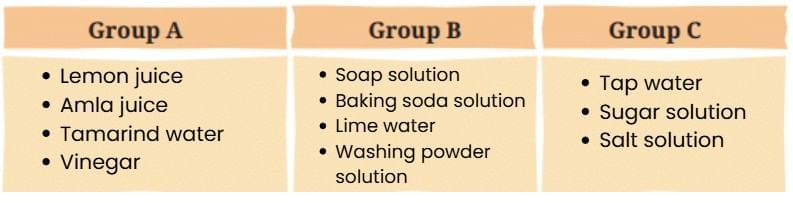
- Group A (Acidic): Lemon juice, amla juice, tamarind water, vinegar
- Group B (Basic): Soap solution, baking soda solution, lime water, washing powder solution
- Group C (Neutral): Tap water, sugar solution, salt solution
Activity 2.2: Let us relate and explore
- Are all the substances in Group A of Table 2.2 edible? Have you ever tasted these edible substances? Can you recall their taste? You will find that all these substances taste sour. Thus, we can say that substances that taste sour tend to contain acids and are acidic in nature.
- Caution - Do not taste anything until asked to do so. Do not taste any unknown substance.
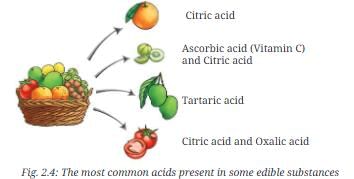
- Some common edible substances and the names of the most common acids present in them are given in Figure 2.4.
 Q. Are all the substances in Group A of Table 2.2 edible?
Q. Are all the substances in Group A of Table 2.2 edible?
Ans: Yes, all substances in Group A (lemon juice, amla juice, tamarind water, vinegar) are edible .
Q. Have you ever tasted these edible substances? Can you recall their taste?
Ans: These substances taste sour, as stated: “You will find that all these substances taste sour”.
Q. Find out and write the names of the most common acids present in the following substances:
- Lemon: Citric acid
- Curd: Lactic acid
- Tamarind: Tartaric acid
- Vinegar: Acetic acid
Explanation: These are common acids associated with these substances, as implied by Fig. 2.4, which lists acids in edible substances.
Q. What do you observe when rubbing baking soda solution between your fingers?
Ans: It feels soapy or slippery.
Q. If litmus is not available, are there some other natural substances that can serve as acid-base indicators?
Ans: Yes, natural substances like red rose extract, turmeric, purple cabbage, red hibiscus, and beetroot can serve as acid-base indicators.
Activity 2.3: Let us prepare
- Collect some fallen petals of red roses available in your surroundings (Fig. 2.5). It is advised not to pluck flowers. You may pick petals or flowers fallen on the ground.
 Fig. 2.5. Red Roses
Fig. 2.5. Red Roses - Take a fistful of the collected petals of red roses and wash them with water.
- Crush the petals using a mortar and pestle.
- Place them in a glass tumbler.
 Fig. 2.6: Red rose petals immersed in hot water
Fig. 2.6: Red rose petals immersed in hot water - Pour some hot water into the glass tumbler to ensure that the crushed flower petals are completely immersed.
- Caution—Perform this step under the supervision of an adult.
- Cover the glass tumbler with a lid. Wait for 5-10 minutes till the water becomes coloured (Fig. 2.6), and filter it.
- The filtrate (liquid after filtration) is the required flower extract (Fig. 2.7) to be used as an acid-base indicator.
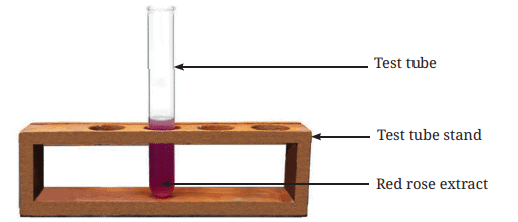 Fig. 2.7: Test tube containing the red rose extract
Fig. 2.7: Test tube containing the red rose extract
Ans: This activity focuses on preparing red rose extract as an acid-base indicator.
Result: The filtrate obtained is a red rose extract that can be used as an acid-base indicator, turning red in acidic solutions and green in basic solutions.
Activity 2.4: Let us find out
- Place 10-20 drops of the prepared red rose extract in each of two small transparent bottles or test tubes. Mark them A and B.
- Add 20-30 drops of lemon juice in test tube A and 20-30 drops of soap solution in test tube B with the help of droppers.
- Observe and record any colour changes (Fig. 2.8) to the extract in Table 2.3.
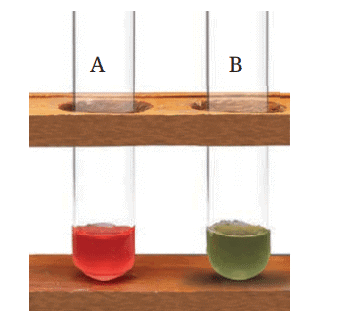 Fig. 2.8: The changes in colour of the red rose extract on adding lemon juice (A) and soap solution (B)
Fig. 2.8: The changes in colour of the red rose extract on adding lemon juice (A) and soap solution (B)
- Repeat the same with the other samples used in Activity 2.1 and record your observations in Table 2.3.
- Discuss your observations with your classmates. Are the samples that change the colour of the flower extract to a shade of red the same as those that changed the colour of blue litmus paper to red? (Group A, Table 2.2).
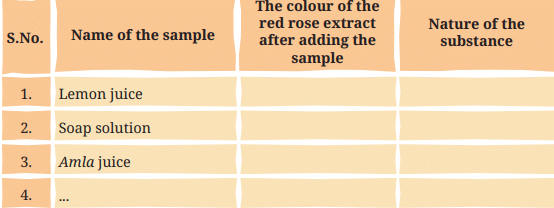 Table 2.3: Testing the nature of samples with the red rose extract
Table 2.3: Testing the nature of samples with the red rose extract
Ans: Colour changes observed:
- Test tube A (lemon juice): The red rose extract turns red.
- Test tube B (soap solution): The red rose extract turns green.
Table 2.3: Testing the nature of samples with the red rose extract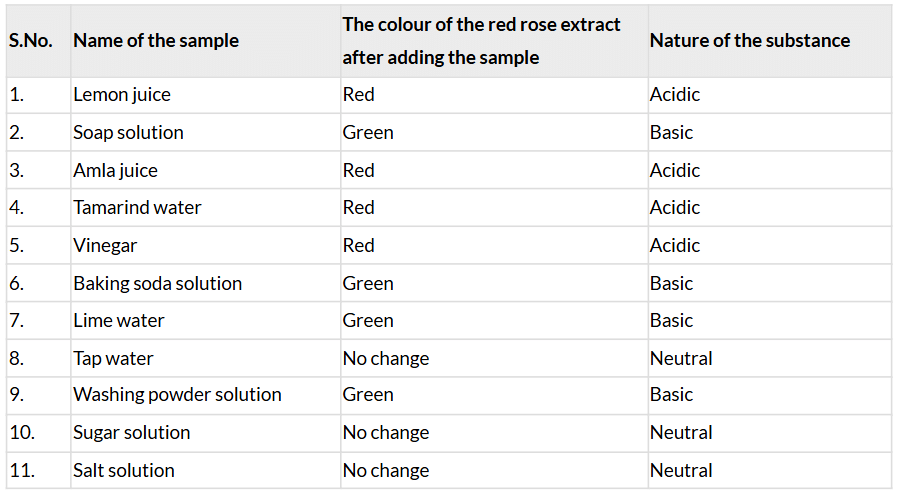
Explanation:
- Red rose extract turns red in acidic solutions (Group A: lemon juice, amla juice, tamarind water, vinegar), green in basic solutions (Group B: soap solution, baking soda solution, lime water, washing powder solution), and shows no change in neutral solutions (Group C: tap water, sugar solution, salt solution).
- The nature of substances is based on Table 2.2.
Q: Are the samples that change the colour of the flower extract to a shade of red the same as those that changed the colour of blue litmus paper to red? (Group A, Table 2.2)
Ans: Yes, lemon juice, amla juice, tamarind water, and vinegar (Group A) turn blue litmus red and red rose extract red.
Q: Are the samples that change the colour of the flower extract to a shade of green the same as those that changed the colour of red litmus paper to blue? (Group B, Table 2.2)
Ans: Yes, soap solution, baking soda solution, lime water, and washing powder solution (Group B) turn red litmus blue and red rose extract green.
Q: Are the samples that do not change the colour of the flower extract the same as those that did not change the colour of red and blue litmus papers? (Group C, Table 2.2)
Ans: Yes, tap water, sugar solution, and salt solution (Group C) don’t change litmus papers or red rose extract.
Q: Can you now fill in the nature of the substances in Table 2.3?
Ans: The nature is filled in the table above (Acidic, Basic, Neutral).
Activity 2.5: Let us prepare
- Take a spoonful of turmeric (haldi) in a petri dish or container and add a little water to make a paste (Fig. 2.9a). You may also grind a piece of fresh turmeric.
- Carefully dip a piece of filter paper in the turmeric paste until it gets yellow colour.
- Take it out and allow it to dry.
- Cut this yellow paper into thin strips, which are used as ‘turmeric paper’ (Fig. 2.9b).
- Caution—Perform this step under the supervision of an adult.
 Fig. 2.9: Preparing turmeric paper
Fig. 2.9: Preparing turmeric paper - Using a dropper, put a drop of each of the samples used in Activity 2.1, one by one, on separate pieces of turmeric paper.
- Record your observations in Table 2.4.
 Table 2.4
Table 2.4 - Based on the observations, we can conclude that turmeric paper can be used to test basic substances. However, it cannot differentiate between acidic and neutral substances.
Ans: Table 2.4: Testing the nature of samples with turmeric paper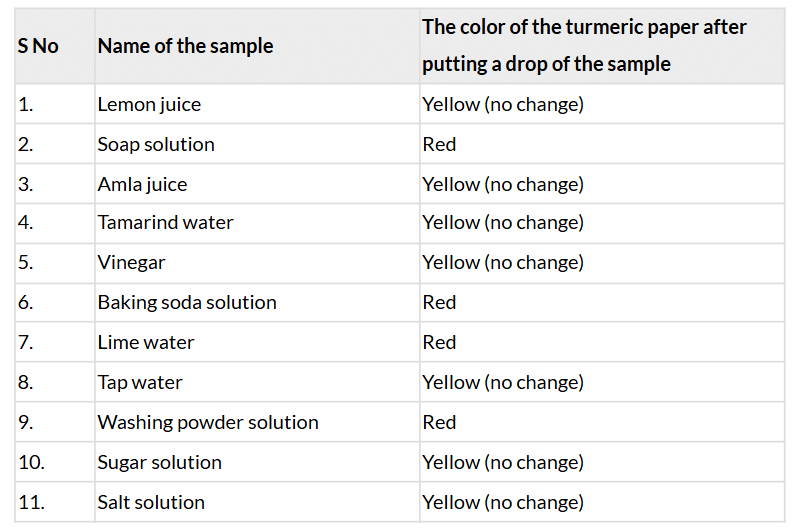
Q: What did you observe?
Ans: Basic substances (soap solution, baking soda solution, lime water, washing powder solution) turn turmeric paper red, while acidic (lemon juice, amla juice, tamarind water, vinegar) and neutral (tap water, sugar solution, salt solution) substances do not change the yellow color.
Q: Do all samples change the colour of the turmeric paper?
Ans: No, only basic substances change the turmeric paper to red; acidic and neutral substances do not.
Q: Group the samples which do not change the colour of the turmeric paper:
Ans: Lemon juice, amla juice, tamarind water, vinegar, tap water, sugar solution, salt solution.
Comparison with Table 2.2: These match Group A (acidic) and Group C (neutral).
Q: Can turmeric paper be used as an indicator for acidic substances?
Ans: No, turmeric paper cannot differentiate acidic substances because it remains yellow for both acidic and neutral substances.
Activity 2.6: Let us investigate
- Take some finely chopped onions in a container, along with some strips of clean cotton cloth or filter paper.
- Tightly close the container and leave it overnight.
- Take two of the cotton cloth or filter paper strips from the container and check their odour.
- Keep them on a clean surface and put a few drops of tamarind water on one strip and a few drops of baking soda solution on the other. Allow the drops to spread on the strips.
- Check the odour again.
Q. Do you notice any change in the odour of the onion strips before and after putting tamarind water and baking soda solution on them?
Ans: Tamarind water (acidic): The onion odor may intensify or change slightly due to the acidic environment, as olfactory indicators like onion change odor in acidic or basic media.
Baking soda solution (basic): The onion odor may diminish or become less pungent, as basic conditions alter the odor differently.
Observations with other substances:
- Acidic substances (e.g., lemon juice, vinegar, amla juice): Similar to tamarind water, these may enhance or alter the onion odor, making it sharper or more noticeable.
- Basic substances (e.g., soap solution, lime water, washing powder solution): Similar to baking soda, these may reduce or change the onion odor to a milder or different scent.
- Neutral substances (e.g., tap water, sugar solution, salt solution): No significant change in odor, as neutral substances don’t affect olfactory indicators.
Explanation: Onion is an olfactory indicator, meaning its odor changes in acidic or basic environments. The exact odor changes aren’t specified, but acidic conditions typically enhance volatile compounds, while basic conditions may neutralize them.
Activity 2.7: Let us experiment
- Take one drop of lemon juice in a test tube and add around twenty drops of water to it. Observe the colour.
- Add a drop of blue litmus solution to it.
- Do you observe any colour change (Fig. 2.10a)?
 Fig. 2.10(a): The colour of the solution on adding blue litmus solution
Fig. 2.10(a): The colour of the solution on adding blue litmus solution - Slowly add drops of lime water to this test tube with the help of a dropper and swirl it well.
 Fig. 2.10(b): The colour of the solution on adding lime water
Fig. 2.10(b): The colour of the solution on adding lime water
Q: Observe the colour (after adding lemon juice and water):
Ans: The solution is colorless or slightly cloudy, as lemon juice diluted with water doesn’t have a strong visible color.
Q: Do you observe any colour change (after adding blue litmus solution)?
Ans: Yes, the blue litmus solution turns red (Fig. 2.10a), because lemon juice is acidic.
Q: What do you observe? Is there any change in the colour of the solution (after adding lime water)?
Ans: As lime water (basic) is added, the solution’s color changes from red to blue (Fig. 2.10b), indicating neutralization.
Q: Can you predict why there is a change in colour (after adding lime water and then lemon juice again)?
Ans: Lime water addition: The acidic lemon juice solution (red with litmus) is neutralized by the basic lime water, making the solution basic, so the litmus turns blue.
Adding lemon juice again: The solution becomes acidic again, turning the litmus red, as the additional acid overcomes the neutralized or basic state.
Explanation: This demonstrates a neutralization reaction where acid (lemon juice) and base (lime water) react to form a neutral solution, but adding more acid shifts it back to acidic.
|
80 videos|224 docs|12 tests
|
FAQs on Class 7 Science Chapter 2 NCERT Based Activity - Exploring Substances: Acidic, Basic and Neutral
| 1. What are acidic, basic, and neutral substances? |  |
| 2. How can we identify whether a substance is acidic, basic, or neutral? |  |
| 3. What are some common examples of acidic and basic substances? |  |
| 4. Why is it important to understand the properties of acidic and basic substances? |  |
| 5. How do acids and bases react with each other? |  |
















