Class 9 Science Chapter 3 Question Answers - Atoms and Molecules
Q1: Define the atomic mass unit.
Ans: One atomic mass unit is a mass unit equal to exactly one-twelfth (1/12th ) the mass of one atom of carbon−12. The relative atomic masses of all elements have been found with respect to an atom of carbon−12.
According to the latest IUPAC (International Union of Pure and Applied Chemistry) recommendations, the atomic mass unit (written as ‘u’ – unified mass) is equal to the mass of one-twelfth (1/12th ) of carbon− 12 atom.
1 amu = 1/12th Mass Of C612
Q2: Write down the formulae of
(a) Sodium oxide
Ans: Sodium oxide – Na2O
(b) Aluminium chloride
Ans: Aluminium chloride – AlCl3
(c) Sodium sulphide
Ans: Sodium sulphide – Na2S
(d) Magnesium hydroxide
Ans: Magnesium hydroxide – Mg(OH)2
Q3: Write down the names of compounds represented the following formulae:
(a) Al2(SO4)3
Ans: Al2(SO4)3 - Aluminium sulphate
(b) CaCl2
Ans: CaCl2 - Calcium chloride
(c) K2SO4
Ans: K2SO4 - Potassium sulphate
(d) KNO3
Ans: KNO3 - Potassium nitrate
(e) CaCO3
Ans: CaCO3 - Calcium carbonate
Q4: What is meant by the term chemical formula?
Ans: The term chemical formula of a compound is said to be the symbolic representation of its composition or it is a notation that shows the type and number of atoms in a molecule of a compound with the help of atomic symbols and numbers.
- They provide information on the elements that constitute the molecules of a compound and the ratio in which the atoms of those elements combine to form the molecules.
- Example: A molecule of water, which is a compound, contains two molecules of hydrogen and one molecule of oxygen. Its chemical formula is H2O
Q5: What are polyatomic ions? Give examples.
Ans: Polyatomic ions are a group of atoms carrying a charge. They are typically clusters of atoms that act as an ion, which carry a fixed charge on them.
Examples:
- Ammonium – NH4+
- Hydroxide – OH−
- Nitrate – NO3−
- Hydrogen carbonate – HCO3-
Q6: Write the chemical formulae of the following.
(a) Magnesium chloride
Ans: Magnesium chloride – MgCl2
(b) Calcium oxide
Ans: Calcium oxide –CaO
(c) Copper nitrate
Ans: Copper nitrate –CuNO3
(d) Aluminium chloride
Ans: Aluminium chloride –AlCl3
(e) Calcium carbonate
Ans: Calcium carbonate –CaCO3
Q7: Give the names of the elements present in the following compounds.
(a) Quick lime
Ans: Quick lime –CaO
Elements present – Calcium, Oxygen
(b) Hydrogen bromide
Ans: Hydrogen bromide –HBr
Elements present – Hydrogen, Bromine
(c) Baking powder
Ans: Baking powder –NaHCO3
Elements present – Sodium, Hydrogen, Carbon, Oxygen
(d) Potassium sulphate
Ans: Potassium sulphate –K2SO4
Elements present – Potassium, Sulphur, Oxygen
Q8: What is the mass of –
Atomic mass of –
S = 32u, Al = 27u, Na = 23u, N = 14u, O = 16u
(a) 1 mole of nitrogen atoms?
Ans: Given its atomic mass, the mass of 1 mole of nitrogen atoms is 14g
(b) 4 moles of aluminium atoms (Atomic mass of aluminium is 27)?
Ans: Given its atomic mass, the mass of 1 mole of aluminium atoms is 27g
Thus, the mass of 4 moles of aluminium atoms is 27 × 4 = 108g
Q9: Convert into mole.
Atomic mass of – C = 12u, H = 1u, O = 16u
(a) 12 g of oxygen gas
Ans: Molar mass of O2 = (16 × 2) = 32g/mole
⇒ 1 mole of O2 = 32g
⇒ 1g of O2 = 1/32 moles
⇒ 12g of O2 = 12 × 1 / 32
= 0.375moles
(b) 20 g of water
Ans: Molar mass of H2O = (1 × 2) + (16 × 1) = 18g/mole
⇒ 1 mole of H2O = 18g
⇒ 1g of H2O = 1/18 moles
⇒ 20g of H2O = 20 × 1/18
=1.11moles
(c) 22 g of carbon dioxide
Ans: Molar mass of CO2 = (12 × 1) + (16 × 2) = 44g/mole
⇒ 1 mole of CO2 = 44g
⇒ 1g of CO2 = 1/44 moles
⇒ 12g of O2 = 22 × 1/44 =0.5moles
Q10: State the Postulates of Dalton Theory?
Ans: Dalton’s atomic theory states that all matter, be it an element, a compound, or a mixture is composed of small particles called atoms.
The postulates of the theory are:
- All matter is made of very tiny particles called atoms, which participate in chemical reactions.
- Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
- Atoms of a given element are identical in mass and chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative number and kinds of atoms are constant in a given compound.
Q11: Find the percentage of water of crystallization in FeSO4.7H2O.
Ans: Atomic mass of –
Fe = 55.9u, S = 32u, H = 1u, O = 16u
Molar mass of FeSO4.7H2O = (55.9 × 1) + (32 × 1) + (16 × 4) + 7 × [(1 × 2) + (16 × 1)]
= 55.9 + 32 + 64 + 7 × [18] = 151.9 + 126 = 227.9g/mole
So, 1 of FeSO4 contains 126/277.6 g water of crystallization.
Converting this fraction into percentage –
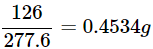 water of crystallization
water of crystallization
Thus, we get 126/277.6 x 100 = 0.4534 x 100 = 45.34%
The percentage of water of crystallization in FeSO4.7H2O is 45.34%.
Q12: 2.42g of copper gave 3.025g of a black oxide of copper, 6.49g of a black oxide, on reduction with hydrogen, gave 5.192g of copper. Show that these figures are in accordance with the law of constant proportion?
Ans: Given:
Case A –
Mass of copper: 2.42g
Mass of copper oxide: 3.025g
Case B –
Mass of black copper oxide: 6.49g
Mass of copper obtained after reduction: 5.192g
Verification: To prove the law of constant proportions, we need to find out the percentage of copper in copper oxide in both cases A and B.
Percentage of copper in Case A = Mass of copper / mass of copper oxide x 100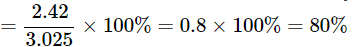
Percentage of copper in Case B = Mass of copper / Mass of copper oxide x100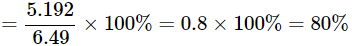
It is clear from the above two calculations that the percentage of copper in copper oxide in both cases A and B is the same. This proves the law of constant proportions – copper always combines with oxygen in the same proportion.
Q13: A compound was found to have the following percentage composition by mass Zn=22.65%, S = 11.15% , H = 4.88% , O = 61.32% . The relative molecular mass is 287g/mole . Find the molecular formula of the compound, assuming that all the hydrogen in the compound is present in water of crystallization.
Ans: Given:
Zn = 22.65%
S = 11.15%
H = 4.88%
O = 61.32%
Relative molecular mass: 287g/mole
To find: Molecular formula of the compound.
Atomic mass of –
Zn = 65.4u, S = 32u, H =1u, O = 16u
To find the formula, we need to find the proportion in which these atoms have combined.
It is known that –
Percentage of element present in a compound
= number of atoms × atomic mass / mass of compound × 100%
⇒ Number of atoms
= percentage of element present in a compound × mass of compound / atomic mass × 100
Using the formula above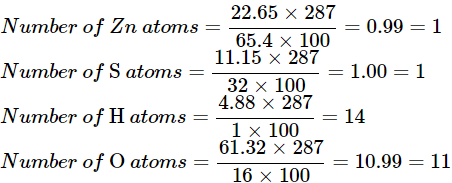
Here, all the Hydrogen atoms belong to the water of crystallization.
Water has the molecular formula – H2O with two molecules of Hydrogen and one molecule of oxygen.
Since we have 14 atoms of Hydrogen, we can say that there are 7 molecules of water in this compound.
That leaves one atom of zinc, one atom of sulphur, and 4 atoms of oxygen (out of 11, 7 atoms of oxygen are in the water of crystallization). It is clear that the compound is Zinc Sulphate with the formula – ZnSO4
Thus, the formula of the compound is ZnSO4.7H2O.
Q14: Which element will be more reactive and why – the element whose atomic number is 10 or the one whose atomic number is 11?
Ans: The element with atomic number 11 is more reactive than the element with atomic number 10.
This is because of the electronic configuration of the atoms.
The element with the atomic number 11, has the configuration of (2,8,1), which means it can easily lose an electron to attain stability. Thus, before losing the electron, it is not stable and is said to be more reactive.
While the element with the atomic number 10, has the configuration of (2,8), which means it is already stable with a completely filled L shell and does not have to gain or lose electrons to attain stability. Thus, it is said to be less reactive.
Q15: What are the failures of Dalton's Atomic theory?
Ans: It does not account for subatomic particles: It stated that atoms were the smallest unit of matter. But, the discovery of subatomic particles namely, protons, electrons, and neutrons disproved this postulate.
It does not account for isotopes: For example hydrogen H11, deuterium H21, and tritiumH31, have the same atomic number, but different mass numbers.
It does not account for isobars. Example: Ar4018 andCa4020, they have different atomic numbers, but the same mass number.
Elements need not combine in simple, whole-number ratios to form compounds: There are complex organic compounds that do not combine in simple ratios of constituent atoms. Example: sugar/sucrose (C11H22O11).
It does not account for allotropes: The differences in the properties of diamond and graphite, even though they contain only carbon, cannot be explained by Dalton’s atomic theory.
Q16: Calculate the Molecular Mass of
Atomic mass of – S = 32u, H = 1u, C = 12u, N = 14u, O =1 6u
(a) Ammonium sulphate (NH4)2SO4
Ans: Molar mass of (NH4)2SO4 = 2 × [(14 × 1) + (1 × 4)] + (32 × 1) + (16 × 4)
= 2 × [(14) + (4)] + (32) + (64) = (2 × 18) + 96 = 36 + 96 = 132g/mole
(b) Penicillin C16H18N2SO4
Ans: Molar mass of C16H18N2SO4 = (12 × 16) + (1 × 18) + (14 × 2) + (32 × 1) + (16 × 4)
= (192)+(18)+(28)+(32)+(64)=334g/mole
(c) Paracetamol C8H9NO
Ans: Molar mass of C8H9NO = (12 × 8) + (1 × 9) + (14 × 1) + (16 × 1)
= (96) + (9) + (14) + (16)=135g/mole
Q17: Write an experiment to show that cathode rays travel in a straight line?
Ans: An experiment to show that cathode rays travel in a straight line can be performed using a fluorescent coated discharge tube and a source of cathode rays, an opaque object, and a high voltage source.
Set-up for the experiment: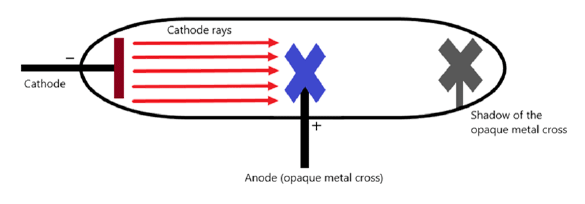
- In a discharge tube coated with a fluorescent substance initiate the production of cathode rays using a high voltage source.
- In the path of the cathode rays, place an opaque object and observe the fluorescence phenomena.
- When cathode rays strike against the screen, they produce fluorescence. But due to the placement of the opaque object, we will observe a sharp shadow being formed on the screen in the shape of the object.
- This shadow of the object can be formed if and only if the cathode rays travel in a straight line and do not bend around the edges of the object.
- This experiment shows that cathode rays travel in a straight line.
Q18: What is radioactivity? What are the applications of radioisotopes?
Ans: Radioactivity is defined as the spontaneous emission of radiation in the form of particles or high-energy photons that are a result of a nuclear reaction. It is the release of energy from the decay of the nucleus of atoms and/or isotopes.
Applications of radioisotopes:
- The isotope of Co−60 emits γ-radiation that is used to treat cancer.
- I−131 is used in the diagnosis and treatment of thyroid gland diseases.
- P−32 is used in the treatment of leukemia and the identification of malignant tumors.
- C−14 is used to study biochemical processes.
Q19: There are two elements C and B. C emits an α – particle and B emits a β – particle. How will the resultant elements charge?
Ans: When an element emits α particle, its atomic number decreases by 2 , and its mass number decreases by 4. This is because alpha particles are positively charged nuclei of Helium with two protons and two neutrons.
- Thus, in the case of element C that emits α particle, its atomic number decreases by 2 and its mass number decreases by 4
- When an element emits β particle, its atomic number increases by 1 and its mass number remains the same. This is because a beta particle is essentially an electron.
- Thus, in the case of element B that emits β particle, its atomic number increases by 1 and its mass number remains the same.
Q20: What are isotopes? Name the isotopes of hydrogen and draw the structure of their atoms.
Ans: Isotopes are defined as the atoms of the same element that have different mass numbers; i.e. elements having the same atomic number but different mass numbers.
Example – Isotopes of Hydrogen:
- Hydrogen H11
- Deuterium H21
- Tritium H31
Structure of Isotopes of Hydrogen: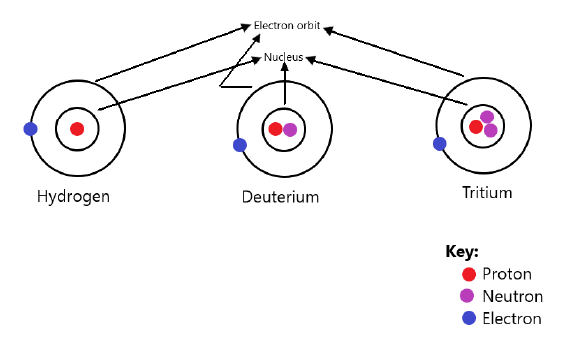
Q21: In a reaction, 5.3g of sodium carbonate reacted with 6g of ethanoic acid. The products were 2.2g of carbon dioxide, 0.9g water and 8.2g of sodium ethanoate. Show that these observations are in agreement with the Law of Conservation of Mass.
Sodium carbonate + Ethanoic acid → Sodium ethanoate + Carbondioxide + Water
Ans: The law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction. This means that the mass of the constituents of a closed chemical reaction will remain the same before and after the reaction.
Mathematically - Mass of reactants = Mass of products
Here, the reactants are Sodium carbonate and Ethanoic acid.
The products are Sodium ethanoate, carbon dioxide and water.
To prove the law of conservation of mass, we need to prove the mass of reactants is equal to the mass of the products.
Given:
Mass of Sodium carbonate: 5.3g
Mass of Ethanoic acid: 6g
Mass of Sodium ethanoate: 8.2g
Mass of Carbon Dioxide: 2.2g
Mass of Water: 0.9g
The reaction –
Sodium carbonate + Ethanoic acid → Sodium ethanoate + Carbondioxide + Water
Now,
Mass of reactants = Mass of Sodium carbonate + Mass of Ethanoic acid
= 5.3 + 6 = 11.3g
Mass of products = Mass of Sodium ethanoate + Mass of carbondioxide + Mass of Water
= 8.2 + 2.2 + 0.9 =11.3g
It is clear from the above calculations that –
Mass of reactants = Mass of products=11.3g
Thus this proves the law of conservation of mass.
Q22: A 0.24g sample of compound of oxygen and boron was found by analysis to contain 0.096g of boron and 0.144g of oxygen. Calculate the percentage composition of the compound by weight.
Ans: Given:
Mass of sample compound: 0.24g
Mass of boron in the sample: 0.096g
Mass of oxygen in the sample: 0.144g
To find: Percentage composition of boron and oxygen in the compound by weight.
Percentage of element present in a compound
=mass of element in compound / mass of compound × 100%
Thus,
Percentage of Boron in compound
= mass of Boron in compound / mass of compound × 100%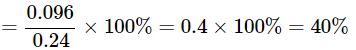
Percentage of Oxygen in compound = mass of Oxygen in compound / mass of compound × 100%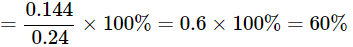
The percentage of Boron by weight in the compound is 40 and the percentage of Oxygen by weight in the compound is 60%.
Q23: If one mole of carbon atoms weighs 12 grams, what is the mass (in grams) of one atom of carbon?
Ans:
It is known that 1 mole of any substance contains 6.023 × 1023 atoms/molecules.
Thus, 1 mole of C = 6.023 × 1023 C-atoms
It is given that one mole of carbon atoms weighs 12 grams
Combining these two observations,
1 mole of C = 12g = 6.023 × 1023 C-atoms
We need to find the mass of one carbon atom.
Since – 12g of C = 6.023 × 1023 C-atoms
i.e. 12 grams contain 6.023 × 1023 C-atoms
Now for the mass of one carbon atom –
6.023 × 1023 C-atoms = 12g of C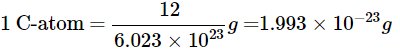
Thus, the mass of one carbon atom is 1.993 × 10−23g
|
84 videos|478 docs|59 tests
|
















