Common ion effect | Chemistry for JEE Main & Advanced PDF Download
Common Ion Effect
If to an ionic equilibrium, AB
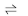
A+ + B‾ , a salt containing a common ion is added, the equilibrium shifts in the backward direction. This is called common Ion effect.
Acetic acid being a weak acid, ionizes to a small extent as:
CH3COOH 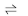
CH3COO‾ + H+
To this solution , suppose the salt of this weak acid with a strong base is added. It ionizes almost completely in the solution as follows and provides the common acetate ions:
CH3COONa ————> CH3COO‾ + Na+
the concentration of CH3COO‾ ions increases and by Le Chatelier’s principle, the dissociation equilibrium shift backwards i.e. dissociation of acetic acid is further suppressed.
2) To the solution of the weak base , NH4OH , NH4Cl is added which provides the common NH4+ ions ,dissociation of NH4OH is suppressed:
NH4OH 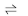 NH4+ +OH‾
NH4+ +OH‾
NH4Cl ———->NH4+ +Cl‾
3) To the solution of silver chloride in water, if NaCl is added which provide the common Cl‾ ions, the solubility AgCl decreases :
AgCl 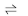 Ag+ + Cl‾
Ag+ + Cl‾
NaCl ——–> Na+ + Cl‾
Increase in the concentration of Cl‾ ions shifts the equilibrium in the backward direction i.e. some solid AgCl separates out.
If to the solution of a weak electrolyte, which ionises to a small extent ,a strong electrolyte having a common ion is added which ionizes almost completely, the ionization of the weak electrolyte is further suppressed .
If to the solution of a sparingly soluble salt, if a soluble salt having a common ion is added ,the solubility of the sparingly soluble salt further decreases.
|
366 videos|853 docs|301 tests
|
FAQs on Common ion effect - Chemistry for JEE Main & Advanced
| 1. What is the common ion effect in chemistry? |  |
| 2. How does the common ion effect affect the solubility of a compound? |  |
| 3. How does the common ion effect relate to the JEE exam? |  |
| 4. Can you provide an example of the common ion effect? |  |
| 5. How can the common ion effect be used to control the solubility of a compound? |  |
















