Class 7 Exam > Class 7 Notes > Year 7 Chemistry (Cambridge) > Elements and the Periodic Table
Elements and the Periodic Table | Year 7 Chemistry (Cambridge) - Class 7 PDF Download
| Table of contents |

|
| Introduction |

|
| Structure of the Periodic Table |

|
| Types of Elements |

|
| Properties and Reactivity |

|
Introduction
- The periodic table is a chart that organizes all known elements.
- Elements are substances that cannot be broken down into simpler substances.
Development of the Periodic Table
- Developed by Dmitry Mendeleev, a Russian scientist.
- Mendeleev noticed similarities between elements and arranged them in columns (groups) and rows (periods).
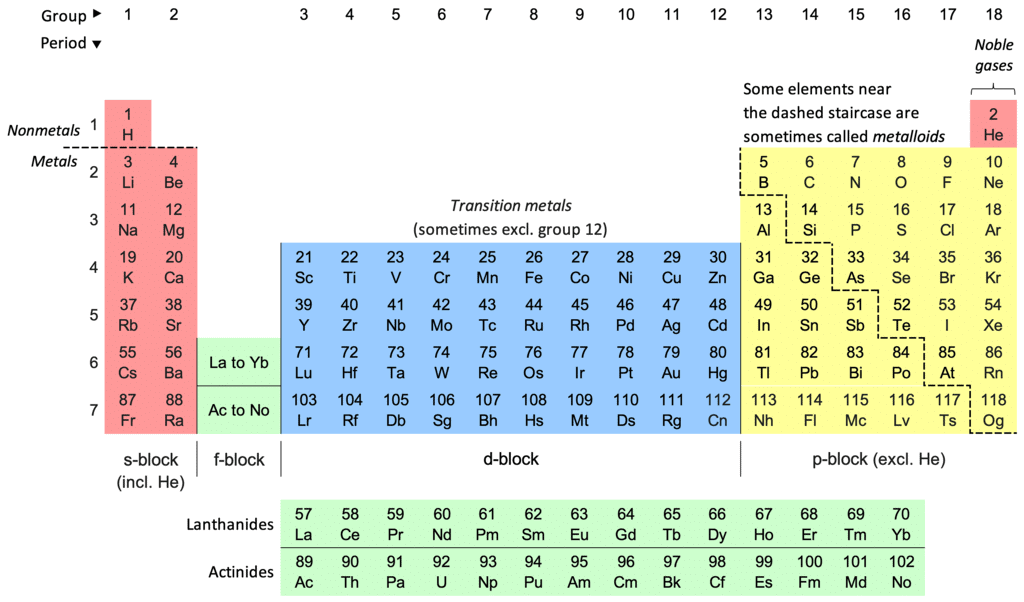
Structure of the Periodic Table
- Groups (Columns):
- Columns are called groups.
- Elements in the same group have similar properties.
- Group 1 elements are known as alkali metals (e.g., lithium, sodium).
- Group 7 elements are known as halogens (e.g., fluorine, chlorine).
- Group 0 (or 8) elements are noble gases (e.g., helium, neon).
- Periods (Rows):
- Rows are called periods.
- Elements in the same period have the same number of electron shells.
Types of Elements
- Metals:
- Found on the left and middle of the periodic table.
- Examples include iron (Fe), aluminium (Al), and lead (Pb).
- Metals are typically shiny, good conductors of electricity, and malleable.
- Non-Metals:
- Found on the right side of the periodic table.
- Examples include oxygen (O), carbon (C), and helium (He).
- Non-metals are generally gases or brittle solids and poor conductors of electricity.
- Metalloids:
- Found along the zigzag line on the periodic table.
- Examples include silicon (Si) and germanium (Ge).
- Metalloids have properties of both metals and non-metals.
Properties and Reactivity
Reactivity Trends:
- Reactivity tends to increase as you move down Group 1 (alkali metals) and decrease as you move down Group 7 (halogens).
- For example, alkali metals become more reactive with water as you move down the group (e.g., lithium to cesium).
Application and Importance
Predicting Reactions:
- The periodic table helps predict how elements will react with each other.
- For instance, knowing that group 1 metals react vigorously with water.
Question for Elements and the Periodic TableTry yourself: Which group of elements is known as alkali metals on the periodic table?View Solution
Conclusion
- Understanding the periodic table is essential in chemistry.
- It helps scientists predict properties and behaviors of elements.
The document Elements and the Periodic Table | Year 7 Chemistry (Cambridge) - Class 7 is a part of the Class 7 Course Year 7 Chemistry (Cambridge).
All you need of Class 7 at this link: Class 7
|
7 videos|15 docs|8 tests
|
FAQs on Elements and the Periodic Table - Year 7 Chemistry (Cambridge) - Class 7
| 1. What are the characteristics of metallic elements on the periodic table? |  |
Ans. Metallic elements on the periodic table are typically shiny, good conductors of heat and electricity, malleable, and ductile.
| 2. What distinguishes non-metallic elements from metallic elements on the periodic table? |  |
Ans. Non-metallic elements on the periodic table are generally poor conductors of heat and electricity, brittle, and not shiny in appearance.
| 3. What are metalloid elements and how do they differ from metallic and non-metallic elements? |  |
Ans. Metalloid elements have properties that are a mixture of metals and non-metals. They exhibit characteristics of both groups, such as being semi-conductors of electricity.
| 4. How are elements arranged on the periodic table in terms of their properties? |  |
Ans. Elements on the periodic table are arranged in order of increasing atomic number, with elements sharing similar properties placed in columns known as groups or families.
| 5. Why is the periodic table important in chemistry and science education? |  |
Ans. The periodic table provides a systematic way to organize and study the properties of elements, helping scientists predict the behavior of elements and compounds. It is a fundamental tool in understanding the building blocks of matter.
Related Searches




















