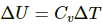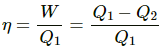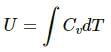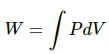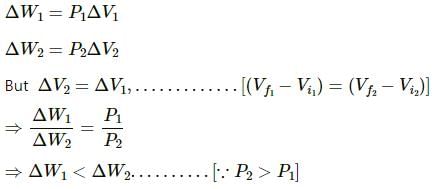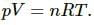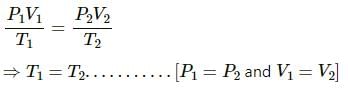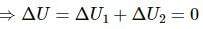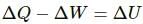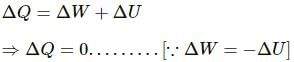HC Verma Questions and Solutions: Chapter 26: Laws of Thermodynamics- 1 | HC Verma Solutions - JEE PDF Download
Short Answers
Q.1. Should the internal energy of a system necessarily increase if heat is added to it?
Change in internal energy of a system,
Here,
Cv = Specific heat at constant volume
ΔT = Change in temperature.
If ΔT = 0, then ΔU = 0, i.e. in isothermal processes, where temperature remains constant, the internal energy doesn't change even on adding heat to the system.
Thus, the internal energy of a system should not necessarily increase if heat is added to it.
Q.2. Should the internal energy of a system necessarily increase if its temperature is increased?
Internal energy of a system increases if its temperature increases. This is valid only for the system of ideal gases and not for all the systems.
For example:- During meting process, temperature of the system remains constant, but internal energy change increases by mL.
⇒ ΔU = mL
Here,
m = Mass of the solid
L = Latent heat of the solid
Q.3. A cylinder containing a gas is lifted from the first floor to the second floor. What is the amount of work done on the gas? What is the amount of work done by the gas? Is the internal energy of the gas increased? Is the temperature of the gas increased?
As a cylinder is lifted from the first floor to the second floor, there is decrease in the atmospheric pressure on the gas and it expands. Therefore, some work is done by the gas on its surroundings. Work done on the gas is zero.
Work done by the gas, W = PΔV (positive)
The increase in the internal energy and temperature of the system will depend on the types of the walls of the system (conducting or insulating).
Q.4. A force F is applied on a block of mass M. The block is displaced through a distance d in the direction of the force. What is the work done by the force on the block? Does the internal energy change because of this work?
If force F is applied on a block of mass M and displacement of block is d, then work done by the force is given by
W = F.d = Fd cos(0°) = Fd
This work done does not change the internal energy of the block as the internal energy does not include the energy due to motion or location of the system as a whole.
Q.5. The outer surface of a cylinder containing a gas is rubbed vigorously by a polishing machine. The cylinder and its gas become warm. Is the energy transferred to the gas heat or work?
As the outer surface of a cylinder containing a gas is rubbed vigorously by a polishing machine, no work is done on the cylinder. Volume of the gas remains constant and the heat energy generated due to friction between the machine and the cylinder gets transferred to the gas as heat energy. This heat energy leads to an increase in the temperature of the cylinder and its gas.
Q.6. When we rub our hands they become warm. Have we supplied heat to the hands?
When we rub our hands, they become warm. In this process, heat is supplied to the hands due to the friction between the hands.
Q.7. A closed bottle contains some liquid. the bottle is shaken vigorously for 5 minutes. It is found that the temperature of the liquid is increased. Is heat transferred to the liquid? Is work done on the liquid? Neglect expansion on heating.
A closed bottle containing some liquid is shaken vigorously such that work is done on the bottle (against the viscous force). Hence, the temperature of the liquid increases. However, no external heat is supplied to the system.
Q.8. The final volume of a system is equal to the initial volume in a certain process. Is the work done by the system necessarily zero? Is it necessarily nonzero?
Work done by the system is neither necessarily zero nor necessarily non-zero.
If in a certain process, the pressure P stays constant, then
ΔW = P Δ V
⇒ W = P(V2 - V1)
As V2 = V1
⇒ W = 0
(Initial volume, V1 = Final volume, V2)
Hence, it is an isobaric process.
Even if P = P(V), net work done will be zero if V2 = V1. In this case, work done is zero.
If the system goes through a cyclic process, then initial volume gets equal to the final volume after one cycle. But work done by the gas is non-zero.
Q.9. Can work be done by a system without changing its volume?
If the system goes through a cyclic process, then initial volume gets equal to the final volume after one cycle. But work done by the gas is non-zero. So, work can be done by a system without changing its volume.
Q.10. An ideal gas is pumped into a rigid container having diathermic walls so that the temperature remains constant. In a certain time interval, the pressure in the container is doubled. Is the internal energy of the contents of the container also doubled in the interval ?
An ideal gas is continuously being pumped into the container. Therefore, the number of moles, n are continuously increasing. In a certain interval,
Pressure, P2 = 2P1
n2 = 2n1
Thus, internal energy, U = nCvT will double as the number of moles get doubled.
Q.11. When a tyre bursts, the air coming out is cooler than the surrounding air. Explain.
When a tyre bursts, adiabatic expansion of air takes place. The pressure inside the tyre is greater than the atmospheric pressure of the surrounding due to which the expansion of air occurs with some work done against the surrounding leading to decrease in the internal energy of the air present inside the tyre. This decrease of internal energy leads to fall in temperature of the inside air. Hence, the air coming out is cooler than that of the surrounding.
Q.12. When we heat an object, it expands. Is work done by the object in this process? Is heat given to the object equal to the increase in its internal energy?
When we heat an object, it expands, i.e. its volume increases.
Work done by the system, ΔW = P Δ V
Using the first law of thermodynamics, we get
ΔQ = ΔU + ΔW
Since the volume changes, ΔW has some non-zero positive value. Thus, heat given to the object is not equal to the increase in the internal energy of the system.
Q.13. When we stir a liquid vigorously, it becomes warm. Is it a reversible process?
When we stir a liquid vigorously, we do work on it due to which its temperature rises and it becomes warm. To bring back the liquid to the initial temperature, heat needs to be extracted from it. But it is an irreversible process that cannot be brought back to the initial state by stirring the liquid again in the opposite direction.
Q.14. What should be the condition for the efficiency of a Carnot engine to be equal to 1?
Efficiency of Carnot engine,
Here,
W = Work done by the engine
Q1 = Heat abstracted from the source
Q2 = Heat transferred to the sink
Thus, forthe total heat which is abstracted from the source gets converted into work.
Q.15. When an object cools down, heat is withdrawn from it. Does the entropy of the object decrease in this process? If yes, is it a violation of the second law of thermodynamics stated in terms of increase in entropy?
When an object cools down, heat is withdrawn from it. Hence, the entropy of the object decreases. But the decrease in entropy leads to the transfer of energy to the surrounding. The second law is not violated here, which states that entropy of the universe always increases as the net entropy increases.
Here,
Net entropy = Entropy of object + Entropy of surrounding
Multiple Choice Questions
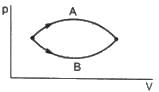
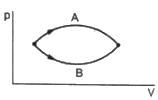

(A) If heat is added to a system, its temperature must increase.
(B) If positive work is done by a system in a thermodynamic process, its volume must increase.

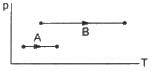
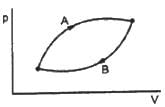
The volumes in the initial states are the same in the two processes and the volumes in the final states are also the same. Let ∆W1 and ∆W2 be the work done by the system in the processes A and B respectively.
|
134 docs
|
FAQs on HC Verma Questions and Solutions: Chapter 26: Laws of Thermodynamics- 1 - HC Verma Solutions - JEE
| 1. What are the laws of thermodynamics? |  |
| 2. How does the first law of thermodynamics relate to energy conservation? |  |
| 3. What is entropy and how does it relate to the second law of thermodynamics? |  |
| 4. What is absolute zero temperature and why is it impossible to reach? |  |
| 5. How does the zeroth law of thermodynamics define thermal equilibrium? |  |