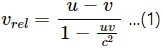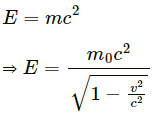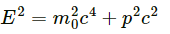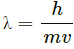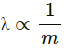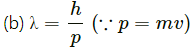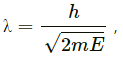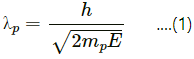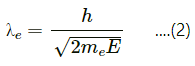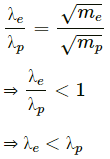HC Verma Questions and Solutions: Chapter 42: Photoelectric Effect and Wave-Particle Duality- 1 | HC Verma Solutions - JEE PDF Download
Short Answers
Q.1. Can we find the mass of a photon by the definition p = mv?
No, we cannot find the mass of a photon by the definition p = mv. The equation p = mv is valid only for objects that move with a velocity that is much slower than the speed of light. The momentum of a relativistic particle like photon is given by
A photon has zero rest mass. Therefore, on putting m = 0 in the equation, we get p = E/c, which is the valid equation for a photon.
Q.2. Is it always true that for two sources of equal intensity, the number of photons emitted in a given time are equal?
Let the source's area be A, and intensity of the source be I. The energy of each emitted photonis E. Then, the number of photons emitted in a given time will be n = I/AE
If the areas of the sources and the wavelengths of light emitted by the two sources are different, then the number of photons emitted will be different.
Q.3. What is the speed of a photon with respect to another photon if (a) the two photons are going in the same direction and (b) they are going in opposite directions?
(a) In relativity, the relative speed of two objects (vrel) moving in the same direction with speeds u and v is given by
As the photons are moving with the speed of light, u = c and v = c.
On substituting the values of u and v in equation (1), we get :
vrel = 0
Thus, relative velocity of a photon with respect to another photon will be 0, when they are going in the same direction.
(b) In relativity, relative speed of two objects moving in opposite directions with speeds u and v is given by...(2)
We know that a photon travels with the speed of light. Therefore, u = c and v = c
On substituting the values of u and v in equation (2), we get :
vrel = c
Thus, the relative velocity of a photon with respect to another photon will be equal to the speed of light when they are going in opposite directions.
Q.4. Can a photon be deflected by an electric field? Or by a magnetic field?
Photons are electrically neutral. Hence, they are not deflected by electric and magnetic fields.
Q.5. A hot body is placed in a closed room maintained at a lower temperature. Is the number of photons in the room increasing?
As the hot body is placed in a closed room maintained at a lower temperature, there will be transfer of heat in the room through convection and radiation. Heat radiation also consists of photons; therefore, photons will be emitted by the hot body. Hence, the number of photons in the room will increase.
Q.6. Should the energy of a photon be called its kinetic energy or its internal energy?
Relativistic equation of energy :
...(1)
Here, p2c2 = kinetic energy of photon
m02c4 = internal energy of photon
We know photons have zero rest mass. Therefore, m0 = 0. Substituting the value of m0 = 0 in equation (1), we get :
E = pc
Thus, the energy of a photon should be called its kinetic energy.
Q.7. In an experiment on photoelectric effect, a photon is incident on an electron from one direction and the photoelectron is emitted almost in the opposite direction. Does this violate the principle of conservation of momentum?
No, it does not violate the principle of conservation of momentum. In the photon-electron collision, the energy and momentum are conserved.
Q.8. It is found that yellow light does not eject photoelectrons from a metal. Is it advisable to try with orange light or with green light?
Photoelectrons are emitted from a metal's surface if the frequency of incident radiation is more than the threshold frequency of the given metal surface. As yellow light does not eject photoelectrons from a metal it means that the threshold frequency of the metal is more than the frequency of yellow light. Since the frequency of orange light is less than the frequency of yellow light, therefore it will not be able to eject photoelectrons from the metal's surface. The frequency of green light is more than the frequency of yellow light. Hence, when it is incident on the metal surface, it will eject electrons from the metal.
Q.9. It is found that photosynthesis starts in certain plants when exposed to sunlight, but it does not start if the plants are exposed only to infrared light. Explain.
Photosynthesis starts when a plant is exposed to visible light. The visible light's photons possess just enough energy to excite the electrons of molecules of the plant without causing damage to its cells. Infrared rays have less frequency than visible light. Due to this, the energy of the photons of infrared rays are not sufficient to initiate photosynthesis. Therefore, photosynthesis does not start if plants are exposed only to infrared light.
Q.10. The threshold wavelength of a metal is λ0. Light of wavelength slightly less than λ0 is incident on an insulated plate made of this metal. It is found that photoelectrons are emitted for some time and after that the emission stops. Explain.
When light of wavelength less than λ0 is incident on the metal surface, the free electrons of the metal will gain energy and come out of the metal surface. As the metal plate is insulated (it is not connected with the battery), the free electrons of the metal will not be replaced by the other electrons. Hence, photoelectron emission will stop after some time.
Q.11. Is p − E/c valid for electrons?
From Einstein's mass- energy equation,
Relativistic momentum,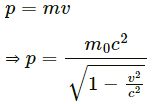
Combining the above equations, we get :
From the above equation, it is clear that for p = E/c to be valid, the rest mass of the body should be zero. As electrons do not have zero rest mass, this equation is not valid for electrons.
Q.12. Consider the de-Broglie wavelength of an electron and a proton. Which wavelength is smaller if the two particles have (a) the same speed (b) the same momentum (c) the same energy?
de-Broglie wavelength,
where h = Planck's constant
m = mass of the particle
v = velocity of the particle
(a) It is given that the speed of an electron and proton are equal.
It is clear from the above equation that
As mass of a proton, mp > mass of an electron, me, the proton will have a smaller wavelength compared to the electron.
So, when the proton and the electron have same momentum, they will have the same wavelength.
(c) de-Broglie wavelength (λ) is also given by
where E = energy of the particle.
Let the energy of the proton and electron be E.
Wavelength of the proton,
Wavelength of the electron,
Dividing (2) by (1), we get :
It is clear that the proton will have smaller wavelength compared to the electron.
Q.13. If an electron has a wavelength, does it also have a colour?
Colour is a characteristic of electromagnetic waves. Electrons behave as a de-Broglie wave because of their velocity. A de-Broglie wave is not an electromagnetic wave and is one dimensional. Hence, no colour is shown by an electron.
Multiple Choice Questions
|
134 docs
|
FAQs on HC Verma Questions and Solutions: Chapter 42: Photoelectric Effect and Wave-Particle Duality- 1 - HC Verma Solutions - JEE
| 1. What is the photoelectric effect? |  |
| 2. How does the photoelectric effect support the wave-particle duality? |  |
| 3. What are the factors that affect the intensity of the photoelectric effect? |  |
| 4. How does the photoelectric effect relate to the concept of energy quantization? |  |
| 5. What are the practical applications of the photoelectric effect? |  |