Important Points: Atoms and Molecules | Science Class 9 PDF Download
| Table of contents |

|
| Laws of Chemical Combination |

|
| Dalton’s Atomic Theory |

|
| What is an Atom? |

|
| What is a Molecule? |

|
| Atomicity |

|
| What is an Ion? |

|
| Molecular Mass |

|
Laws of Chemical Combination
The chemical reaction between two or more substances giving rise to products is governed by certain laws. These laws are called Laws of Chemical Combination’.
We will study the following laws in higher classes:
- Law of Multiple Proportion
- Gay Lussac’s law of combining volume
Law of Conservation of Mass
- According to this law, “Mass can neither be created nor destroyed.” In a chemical reaction, this law can be understood in the following way.
- During a chemical reaction total mass of reactants will be equal to the total mass of products.”
- For example,( Reactant ) A + B → AB ( Product )
Law of Constant Proportion
According to this law, “A pure chemical compound always contains the same elements combined together in the same proportion by mass irrespective of the fact from where the sample has been taken or from which procedure has it been produced.”
Dalton’s Atomic Theory
Dalton's Atomic Theory is a fundamental concept in chemistry that helps us understand the behavior of matter. It was proposed by the British scientist John Dalton in the early 19th century. This theory is built upon some key ideas, or postulates, which are easy to grasp for students like you. Let's break them down:
Postulates of Dalton’s Atomic Theory
1. Tiny Building Blocks: Everything around us is made up of incredibly small particles known as "atoms." These atoms are the basic units of matter.
2. Unbreakable and Indestructible: Atoms are like the LEGO blocks of the universe. They cannot be divided into smaller parts, and they can't be created or destroyed during chemical reactions. This idea connects to the "Law of Conservation of Mass," which says that matter is neither created nor destroyed in a chemical reaction; it just changes form.
3. Identical Atoms: Atoms of the same element are identical to one another. In other words, all the atoms of, say, oxygen are the same in terms of mass and chemical behavior.
4. Different Atoms: Atoms of different elements, like oxygen and carbon, are not the same. They have different masses and behave differently in chemical reactions.
5. Simple Ratios: Atoms like to combine with other atoms to make compounds. When they do, they do it in simple ratios. This postulate supports the "Law of Constant Composition," which means that a compound always contains the same elements in the same proportions.
6. Steady in Compounds: In compounds, the types and numbers of atoms always stay constant. So, if you have a molecule of water (H2O), it will always have two hydrogen atoms and one oxygen atom.
What is an Atom?
- According to modern atomic theory, an atom is the smallest particle of an element that takes part in a chemical reaction such that during the chemical reaction, the atom maintains its identity, throughout the chemical or physical change.
- Atoms are very small and hence can’t be seen even through a very powerful microscope.
Measurement of Atomic radius
- 1 nm = 109m
- The atomic radius of the smallest atom in hydrogen is 0.37 x 10-10m or 0.037 nm.
What is a Molecule?
- A molecule is a group of two or more atoms that are chemically bonded with each other.
- A molecule is the smallest particle of matter (except element) which is capable of an independent existence and show all properties of that substance.
- E.g., ‘H2O’ is the smallest particle of water which shows all the properties of water.
Atomicity
Atomicity refers to the number of atoms of an element that constitute a single molecule.
It indicates how many atoms are chemically bonded together to form a stable and independent unit. Atomicity is a property that varies among different elements and compounds.
What is an Ion?
- The charged particles (atoms) are called ions, they are formed by attaining positive charge or negative charge on it.
- A negatively charged ion is called anion (Cl–).
- A positively charged ion is called cation (Na+).
 |
Download the notes
Important Points: Atoms and Molecules
|
Download as PDF |
Valency
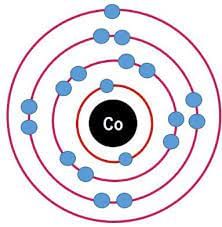
The combining capacity of an element is known as its valency. Valency is used to find out how the atom of an element will combine with the atom of another element to form a chemical compound.
(Every atom wants to become stable, to do so it may lose, gain or share electrons.)
- If an atom consists of 1, 2 or 3 electrons in its valence shell then its valency is 1, 2 or 3 respectively,
- If an atom consists of 5, 6 or 7 electrons in the outermost shell, then it will gain 3, 2 or 1 electron respectively and its valency will be 3, 2 or 1 respectively.
- If an atom has 4 electrons in the outermost shell than it will share this electron and hence its valency will be 4.
- If an atom has 8 electrons in the outermost electron and hence its valency will be 0.
Writing Chemical Formulae
It is the symbolic representation of the composition of a compound.
Characteristics of Chemical Formulae
- The valencies or charges on ion must balance.
- When a compound is formed of metal and non-metal, a symbol of metal comes first. E.g., CaO, NaCl, CuO.
- When polyatomic ions are used, the ions are enclosed in brackets before writing the number to show the ratio. E.g., Ca(OH)2, (NH4)2 SO4
Rules for Writing Chemical Formulae
Rule 1: We first write symbols of elements that form a compound.
Rule 2: Below the symbol of each element, we should write their valency.
Rule 3: Now cross over the valencies of combining atoms.
Rule 4: With the first atom, we write the valency of the second atom (as a subscript).
Rule 5: With the second atom, we write the valency of the first atom (subscript).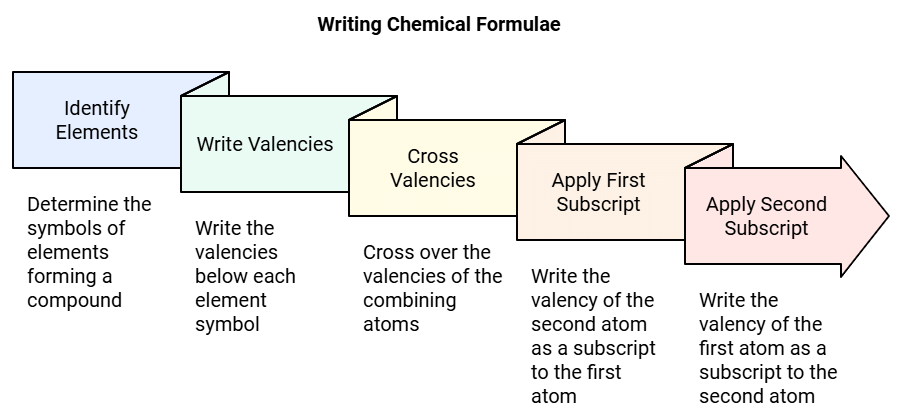
Molecular Mass
The molecular mass of a substance is the total sum of the atomic masses of all the atoms present in a molecule of that substance. It is measured in atomic mass units (u).
example: Relative Molecular Mass of Water (H₂O):
- The atomic mass of hydrogen (H) = 1u, atomic mass of oxygen (O) = 16u.
- Molecular mass of water (2H + 1O) = 2 × 1 + 1 × 16 = 18u.
Formula Unit Mass
Formula unit mass is calculated similarly to molecular mass but applies to substances with ions as constituent particles. It represents the sum of the atomic masses of all atoms in a formula unit of a compound.
|
84 videos|384 docs|61 tests
|
FAQs on Important Points: Atoms and Molecules - Science Class 9
| 1. What are the main laws of chemical combination? |  |
| 2. How did Dalton’s Atomic Theory contribute to modern chemistry? |  |
| 3. What is the difference between an atom and a molecule? |  |
| 4. What is atomicity, and how is it determined? |  |
| 5. What is the significance of molecular mass in chemistry? |  |






















