Integer Answer Type Questions: Modern Physics | JEE Advanced | 35 Years Chapter wise Previous Year Solved Papers for JEE PDF Download
Q.1. An α-particle and a proton are accelerated from rest by a potential difference of 100 V. After this, their de Broglie wavelengths are λα and λp respectively. The ratio 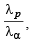 to the nearest integer, is (2010)
to the nearest integer, is (2010)
Ans. 3
Solution. 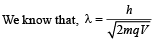
Q.2. To determine the half life of a radioactive element, a student plots a graph of 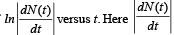 is the rate of radioactive decay at time t. If the number of radioactive nuclei of this element decreases by a factor of p after 4.16 years, the value of p is (2010)
is the rate of radioactive decay at time t. If the number of radioactive nuclei of this element decreases by a factor of p after 4.16 years, the value of p is (2010)
Ans. 8
Solution. We know that N = N0e-λt
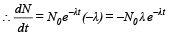
Taking log on both sides
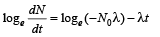
Comparing it with the graph line,
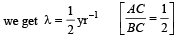
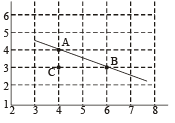
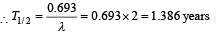
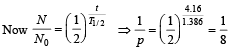
Q.3. The activity of a freshly prepared radioactive sample is 1010 disintegrations per second, whose mean life is 109 s. The mass of an atom of this radioisotope is 10–25 kg. The mass (in mg) of the radioactive sample is (2011)
Ans. 1
Solution.
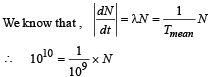
∴ N = 1019
i.e. 1019 radioactive atoms are present in the freshly prepared sample.
The mass of the sample = 1019 × 10–25 kg = 10–6kg = 1 mg
Q.4. A silver sphere of radius 1 cm and work function 4.7 eV is suspended from an insulating thread in freespace. It is under continuous illumination of 200 nm wavelength light. As photoelectrons are emitted, the sphere gets charged and acquires a potential. The maximum number of photoelectrons emitted from the sphere is A × 10z (where 1 < A < 10). The value of ‘z’ is (2011)
Ans. 7
Solution. Stopping potential 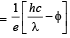 where hc = 1240eV –nm
where hc = 1240eV –nm
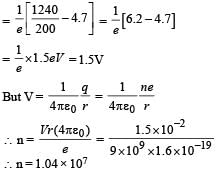
Comparing it with A × 10z we get, z = 7
Q.5. A proton is fired from very far away towards a nucleus with charge Q = 120 e, where e is the electronic charge.
It makes a closest approach of 10 fm to the nucleus. The de Broglie wavelength (in units of fm) of the proton at its start is: (take the proton mass, mp = (5/3) × 10 – 27 kg; h/e = 4.2 × 10–15 J.s / C; 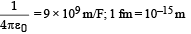 (2012- I)
(2012- I)
Ans. 7
Solution. Loss in K.E. of proton = Gain in potential energy of the proton – nucleus system
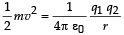
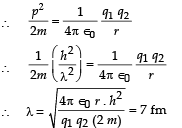
Q.6. The work functions of Silver and Sodium are 4.6 and 2.3 eV, respectively. The ratio of the slope of the stopping potential versus frequency plot for Silver to that of Sodium is (JEE Adv. 2013-I)
Ans. 1
Solution. For photoelectric effect
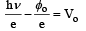
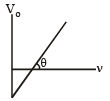
The slope is
tan θ = h/e = constant
∴ The ratio will be 1.
Q.7. A freshly prepared sample of a radioisotope of half-life 1386 s has activity 103 disintegrations per second. Given that ln2 = 0.693, the fraction of the initial number of nuclei (expressed in nearest integer percentage) that will decay in the first 80 s after preparation of the sample is (JEE Adv. 2013-I)
Ans. 4
Solution. For a radioactive decay
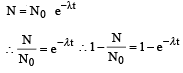
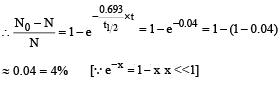
Q.8. A nuclear power plant supplying electrical power to a village uses a radioactive material of half life T years as the fuel. The amount of fuel at the beginning is such that the total power requirement of the village is 12.5% of the electrical power available from the plant at that time. If the plant is able to meet the total power needs of the village for a maximum period of nT years, then the value of n is (JEE Adv. 2015)
Ans. 3
Solution.
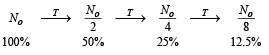
Three half life are required. Therefore n = 3
Q.9. Consider a hydrogen atom with its electron in the nth orbital.
An electromagnetic radiation of wavelength 90 nm is used to ionize the atom. If the kinetic energy of the ejected electron is 10.4 eV, then the value of n is (hc = 1242 eV nm) (JEE Adv. 2015)
Ans. 2
Solution. 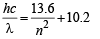
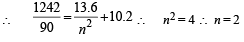
Q.10. For a radioactive material, its activity A and rate of change of its activity R are defined as 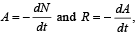 where N(t) is the number of nuclei at time t. Two radioactive sources P (mean life τ) and Q (mean life 2τ) have the same activity at t = 0. Their rates of change of activities at t = 2τ are RP and RQ, respectively.
where N(t) is the number of nuclei at time t. Two radioactive sources P (mean life τ) and Q (mean life 2τ) have the same activity at t = 0. Their rates of change of activities at t = 2τ are RP and RQ, respectively. 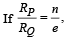 then the value of n is (JEE Adv. 2015)
then the value of n is (JEE Adv. 2015)
Ans. 2
Solution.
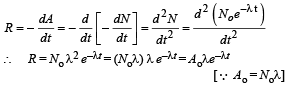
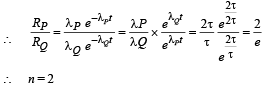
Q.11. An electron is an excited state of Li2+ ion has angular momentum 3h/2π. The de Broglie wavelength of the electron in this state is pπ a0 (where a0 is the Bohr radius). The value of p is (JEE Adv. 2015)
Ans. 2
Solution. Given mvr = 3h/2π ⇒ n = 3
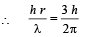
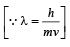
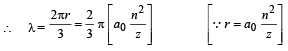
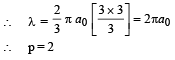
Q.12. The isotope 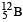 having a mass 12.014 u undergoes β–decay to
having a mass 12.014 u undergoes β–decay to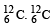 has an excited state of the nucleus
has an excited state of the nucleus 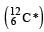 at 4.041 MeV above its ground state.
at 4.041 MeV above its ground state.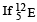 decays to
decays to 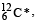 the maximum kinetic energy of the β–particle in units of MeV is (1 u = 931.5 MeV/c2, where c is the speed of light in vacuum). (JEE Adv. 2016)
the maximum kinetic energy of the β–particle in units of MeV is (1 u = 931.5 MeV/c2, where c is the speed of light in vacuum). (JEE Adv. 2016)
Ans. 9
Solution. Maximum kinetic energy of β-particle
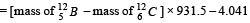
= [12.014 – 12] × 931.5 – 4.041] = 9MeV
Q.13. A hydrogen atom in its ground state is irradiated by light of wavelength 970 Å. Taking hc/e = 1.237 × 10–6 eV m and the ground state energy of hydrogen atom as –13.6 eV, the number of lines present in the emission spectrum is (JEE Adv. 2016)
Ans. 6
Solution. 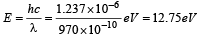
∴ The energy of electron after absorbing this photon
= –13.6 + 12.75 = – 0.85eV
This corresponds to n = 4
Number of spectral line 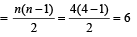
|
347 docs|185 tests
|
FAQs on Integer Answer Type Questions: Modern Physics - JEE Advanced - 35 Years Chapter wise Previous Year Solved Papers for JEE
| 1. What are the key topics covered in the Modern Physics section of the JEE Advanced exam? |  |
| 2. How important is the Modern Physics section in the JEE Advanced exam? |  |
| 3. How can I effectively prepare for the Modern Physics section of the JEE Advanced exam? |  |
| 4. What are some common mistakes students make in the Modern Physics section of the JEE Advanced exam? |  |
| 5. Are there any specific tips to improve performance in the Modern Physics section of the JEE Advanced exam? |  |
















