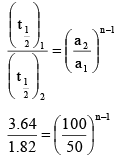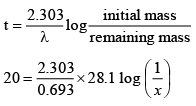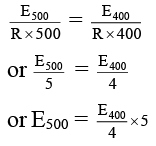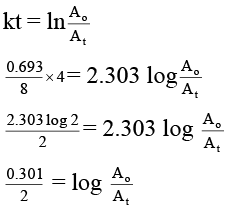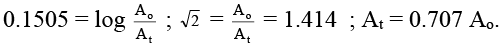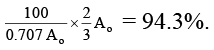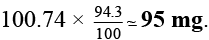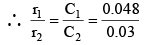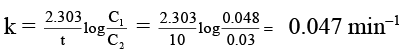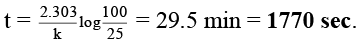Integer Answer Type Questions for JEE: Chemical Kinetics & Nuclear Chemistry | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. The following data were obtained at a certain temperature for the decomposition of ammonia in contact with tungsten:
Find the order of the reaction.
Ans. 2
We know,
2 = 2n -1
⇒ n - 1 = 1
n = 2
∴ Order of the reaction = 2.
Q.2. One of the hazards of nuclear explosion is the generation of 90Sr and its subsequent incorporation in bones. This nuclide has a half-life of 28.1 years. Suppose one microgram was absorbed by a new-born child, how much 90Sr in nanograms will remain in his bones after 20 years? Use: pH of 0.61 M HCl is approximately 0.214.
Ans. 610
⇒ x = 0.61 μg(as it is given -log 0.61 = 0.214)
⇒ 610 x 10-9g = 610 nanograms.
Q.3. A hydrogenation reaction is carried out at 500 K. If the same reaction is carried out in presence of a catalyst at the same rate, the temperature required is 400 K. Calculate the activation energy of the reaction if the catalyst lowers the activation barrier by 25 kJ mol-1.
Ans. 125
Arrhenius equation may be given as, k = Ae-Ea/RT
Let k500 and k400 be the rate constants at temperatures 500 K and 400 K (in presence of catalyst) respectively. E500 and E400 be the activation energies at temperatures 500 K and 400 K respectively.
k500 = Ae -E500 / Rx500 …(i)
k400 = Ae -E400 / Rx500 …(ii)
Given k500 = k400 (same rates in presence and absence of a catalyst).
On comparing equation (i) with equation (ii),
Given, E500 = E400 + 25
Substituting equation (iii)
E400 + 25 = E400 × 1.25
or E400 = 25/0.25 = 100 kJ mol-1
So, E500 = 100 + 25 = 125 kJ mol-1.
Q.4. A sample of 53I131, as iodide ion was administered to a patient as a carrier consisting of 100.74 mg of stable iodide ion. After 4 days, 66.66 % of the initial radioactivity was detected in the thyroid gland of the patient. What is the approximate mass of the stable iodide ion in mg that has migrated to the thyroid gland? Assume that the rate of migration of stable iodide ion is same as that of radioactive iodide. [t1/2 of 131I = 8.0 days] Given: log 2 = 0.301 and antilog of 0.1505 = √2.
Ans. 95
According to decay law, the radioactivity of the sample can be determined (but this takes into account only the less of the sample due to decay) as
While the observed activity given takes into account the loss due to decay as well as the loss due to migration of radioactive iodide ion. So, if activity after 4 days would have been 0.707 Ao, the migration will be 100%.
But when the activity after 4 days is 0.6666 Ao orthe migration will be
As given in the problem, the rate of migration of stable iodide ion is same as that of radioactive iodide, so the mass of stable iodide ion that has migrated to thyroid gland will be
Q.5. The rate of a first-order reaction is 0.048 mol litre–1 s–1 at 10 minutes and 0.03 mol litre–1 s–1 at 20 minutes after initiation. Find the time in seconds when 75% of the reactant is consumed.
Ans. 1770
r1 = kC1, and r2 = kC2
|
446 docs|929 tests
|