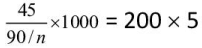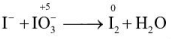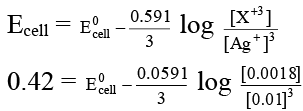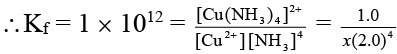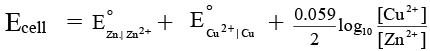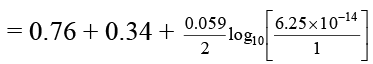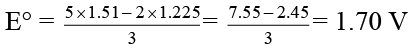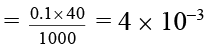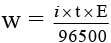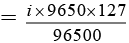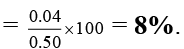Integer Answer Type Questions for JEE: Electrochemistry | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. For the reaction Ag → 5B(g). It initially 2 moles of A are present. What is ratio of total no of moles after 25% reaction is completed to initial no. of moles.
Ans. 2
A(g) → 5B(g)
2 moles 0
2 - 0.5 2.5 total no. of moles 1.5 + 2.5 = 4
Total of moles/Initial of moles = 4/2 = 2
Q.2. 5.9 gm of a sample of bleaching powder is treated with excess acetic acid and Kl solution. The liberated l2 required 50 ml of M/10 hypo. What is the percent of available chlorine in the sample.
Ans. 3
Meq. of bleaching powder = Meq. of Cl2 = Meq. of hypo
w/35.5 × 1000 = 50 × 1/10
wCl2 =0.1775g
∴ % chlorine = 0.1775/5.9 × 100 = 3
Q.3. 45 g of acid of mol. wt. 90 neutralized by 200 ml. of 5 N caustic potash. What is the basicity of the acid?
Ans. 2
meq. of acid = meq. of caustic potash
∴
∴ n = 2
Q.4. To make 0.01 N solution of a salt from its 0.1 N, 1 litre solution, the amount of H20 required is
Ans. 9
0.1 N × 1 = 0.01 × V
V = 0.1/0.01 = 10 litre
∴ volume of H20 required = 10 -1 = 9
Q.5. What is the n factor of IO-3 in the given equation I- + IO-3 → I2 + H2O
Ans. 5
N factor = 5
Q.6. The charge on cobalt in [Co (CN )6]3- is
Ans. 3
In [Co (CN )6]3- complex Co shows + 3 oxidation state.
Q.7. To find the standard potential of X+3 | X electrode, the following cell is constituted
X(s) | X3+ (0.0018 M) || Ag+1(0.01 M) | Ag(s)
The emf of this cell is found to be 0.42 volt. Calculate the standard potential of the half-cell reaction
X+3 + 3e → X.
Express your answer in millivolts.
(Given log 2 = 0.3010, log 3 = 0.4771 and 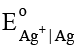 = 0.8V)
= 0.8V)
Ans. 316
X + 3Ag+ → 3 Ag + X+3
0.42 = E0cell – 0.064
E0cell = 0.484 volt
E0anode = E0cathode - E0cell
E0anode = [0.80 – .484] = 0.316 V
= 316 millivolt.
Q.8. A Daniel cell originally has 1.0 M Zn2+ in the anode half-cell and 1.0 M Cu2+ in the cathode half-cell. Estimate the cell potential of the Daniel cell in milli volt after sufficient ammonia has been added to the cathode compartment to make the NH3 concentration 2.0 M. Given that 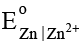 = 0.76 V and
= 0.76 V and 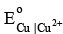 = -0.34 V. Also equilibrium constant for the formation of [Cu(NH3)4]2+ is 1 × 1012.
= -0.34 V. Also equilibrium constant for the formation of [Cu(NH3)4]2+ is 1 × 1012.
Ans. 710 mV
Cu2+ + 4NH3 ⇌ [Cu(NH3)4]2+
∴ x = 6.25 × 10-14 M
Note that due to high value of Kf almost all of the Cu2+ ions are converted to Cu (NH3)2+4 ion.
Now,
Ecell= 0.71 V = 710 mV.
Q.9. What is standard reduction potential in millivolt for the half cell Pt | MnO-4, MnO2?
Given: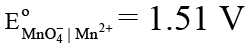
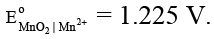
Ans. 1700
MnO-4 + 8H+ + 5e- → Mn2+ + 4H2O E° = 1.51 V
MnO2 + 4H+ + 2e– → Mn2+ + 2H2O E° = 1.225 V.
Subtracting MnO-4 + 4H+ + 3e- → MnO2 + 2H2O
E° = ?
= 1700 millivolt.
Q.10. In the electrolysis of KI, I2 is formed at the anode by the reaction;
2I- → I2 + 2e-
After the passage of current of 0.5 ampere for 9650 seconds, I2 formed required 40 ml of 0.1 M Na2S2O3.5H2O solution in the reaction;
I2 + 2S2O2-3 → S4O2-6 + 2I-
What is the current efficiency?
Ans. 8%
Number of moles of hypo = MV/1000
∴ Number of moles of I2 = 2 × 10-3
Mass of I2 = 2 × 10-3 × 254 g
2 × 10-3 × 254
i = 0.04 ampere
Current efficiency
|
446 docs|929 tests
|