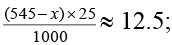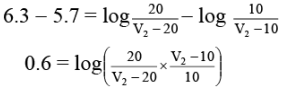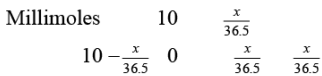Integer Answer Type Questions for JEE: Equilibrium | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. A solution of HCOOH is titrated with KOH solution. When half of the acid is neutralised, AgCN is added to make a saturated solution. Calculate the solubility of AgCN in milligram per 100 litres of solution. Ka (HCN) = 10–10, Ksp (AgCN) = 10–16, Ka (HCOOH) = 10–4.
Ans. 134 mg / 100 L
pH of buffer = pKa + log [Salt/Acid] = 4 + log 1/1 = 4
AgCN ⇌ Ag+ + CN– x is solubility of AgCN
Hence,
⇒ [CN–] = 10–6x = 10–16
Ksp = x × 10–6x
⇒ x2 = 10–10 ⇒ x = 10–5 M
Hence, the solubility of AgCN is 10–5 M = 134 mg / 100 L.
Q.2. What volume in ml of 0.10 M sodium formate solution should be added to 50 ml of 0.05 M formic acid to produce a buffer solution of pH 4.1 pKa for formic acid is 3.80.
Ans. 50 ml.
Let x ml of 0.10 M sodium formate be added.
∴ Moles of HCOONa added = 0.1x × 10-3
Moles of HCOOH = 0.05 × 50 × 10-3 = 2.5 × 10-3
For acidic buffer, pH = pKa + log [salt/acid]
4.1 = 3.8 + log 0.04x.
On solving, x = 50 ml.
Q.3. 500 ml of 0.150 M AgNO3 solution is mixed with 500 ml of 1.09 M Fe2+ solution and the reaction is allowed to reach equilibrium at 25°C.
Ag+(aq) + Fe2+(aq) ⇌ Fe3+(aq) + Ag(s)
For 25 ml of the equilibrium solution, 30 ml of 0.0833 M KMnO4 were required for oxidation. Calculate the approximate equilibrium constant for the reaction at 25°C.
Ans. 3.0
Ag+(aq) + Fe2+(aq) ⇌ Ag(s) + Fe3+(aq)
No. of m moles initially 500 × 0.15 500 × 1.09 0 0
= 75 =545
M moles at equilib. 75 -x 545 - x x x
On titration of reaction mixture with KMnO4, only Fe2+ reacts with it.
∴ Equivalents of Fe2+ in 25 ml = Equivalents of KMnO4
= 30 × 10-3 × 0.0833 × 5
545 -x = 500;
x = 45
Q.4. An unknown volume and unknown concentration of weak acid HX is titrated with NaOH of unknown concentration. After addition of 10.0 cm3 of NaOH solution, pH of solution is 5.7 and after the addition of 20.0 cm3 of NaOH solution, the pH is 6.3. Calculate the pKa for the weak acid, HX. (Given: antilog of 0.6 ≈ 4)
Ans. 6.0
Let the molarity and volume of HX be M1 and V1 ml respectively while the molarity of NaOH be M2.
of 10 c.c of NaOH
Since, weak acid HX and NaX are left after the reaction they will constitute an acidic buffer.…(1)
Let V2 ml be the volume of NaOH required to neutralize given HX completely.
∴ At equivalence point, M1V1 = M2V2
Dividing the numerator and denominator of log term by M2, we get
Similarly, after the addition of 20 c.c of NaOH, we have
Subtracting equation (2) from equation (3)
Taking antilog,
∴ V2 = 30 ml.
Putting V2 in equation (2),
pKa = 5.7 + log 2 = 5.7 + 0.3 = 6.0.
Q.5. Calculate the weight in mg of HCl added to 100 ml of 0.1 N BOH to have its pH = 6.6 and Kb = 6.25 × 10-8 ; Antilog (-7.4) = 3.98 × 10-8. (Assume there is no change in volume on addition of HCl)
Ans. 223 mg.
Let the mass of HCl added be x mg
BOH + HCl → BCl + H2O
This constitutes basic buffer. For basic buffer, the [OH-] is given by
On solving, n = 223
∴ Weight of HCl required = 223 mg.
|
446 docs|929 tests
|