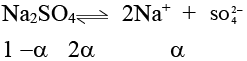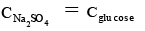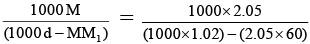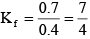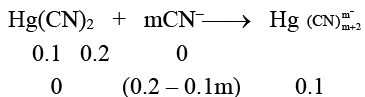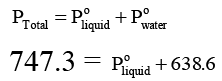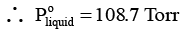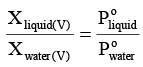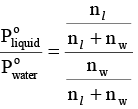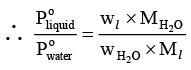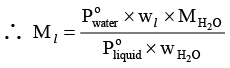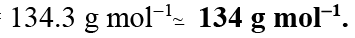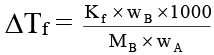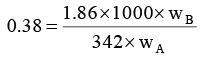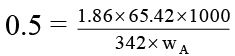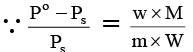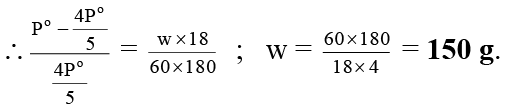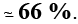Integer Answer Type Questions for JEE: Solutions | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. At 300 K two liquids A and B forms an ideal solution. The vapour pressure of pure A and B are 400 mm and 600 mm respectively. If 1 mol of A and x moles of B are mixed, vapour pressure of solution becomes 550 mm. What is value of x
Ans. 3
∴ (1/(1+x)).400 + (x/(1+x)). 600
⇒ 400 + 600x = 550 + 550 x
⇒ 50 x = 150, x = 3
Q.2. The amount of urea to be dissolved in 500 ml of water (K = 1.86) to produce a depression of 1.86°C in freezing point is 10x gram. What is the value of x.
Ans. 3
ΔTf = Kfm
⇒ 1.86 = 1.83 m ⇒ m = 1
For one molal solution amount of urea, CO(NH2)2, to be dissolved in 500 g H2O is 30 gm
∴ 10x = 30 ⇒ x = 3
Q.3. Elevation in boiling point of a molar glucose solution (density = 1.2 g/ml) is
Ans. 0.98 kb
Molality of 1 < glucose solution =
0.98.
ΔTb = kb × m = 0.98 kb.
Q.4. 0.04 M Na2SO4 solution is isotonic with 0.1 M glucose at the same temperature. What is the apparent degree of dissociation of Na2SO4?
Ans. 0.75
At the same temperature, isotonic solutions will have same concentrations.
∴
0.04(1 + 2α) = 0.1
α = 0.75
Q.5. Density of a 2.05 M solution of acetic acid in water is 1.02 g/ml. The molality of the solution is (Atomic mass: H = 1, C = 12, O = 16)
Ans. 2.28 mol kg–1
Molality =
= 2.28 mol kg-1.
Q.6. Freezing point of 0.2 M KCN solution is –0.7°C. On adding 0.1 mole of Hg(CN)2 to one litre of the 0.2 M KCN solution, the freezing point of the solution becomes – 0.53°C due to the reaction Hg(CN)2 + mCN– → Hg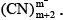 What is the value of m assuming molality = molarity?
What is the value of m assuming molality = molarity?
Ans. 2
0.7 = 2 x Kf x 0.2 ⇒
Now, Molarity of K+ = 0.2 M
Molarity of CN– = (0.2 – 0.1m) M
Molarity of complex = 0.1 M
0.53 = Kf (0.2 + 0.2 – 0.1m + 0.1) = Kf (0.5 – 0.1m)
⇒ 0.53 = (7/4) (0.5 – 0.1m)
⇒ 2.1 = 3.5 – 0.7m
⇒ 0.7m = 1.4
⇒ m = 2.
Q.7. When a liquid that is immiscible with water was steam distilled at 95.2°C at a total pressure of 747.3 Torr, the distillate contained 1.27 g of the liquid per gram of water. Calculate the molar mass of the liquid. The vapour pressure of water is 638.6 Torr at 95.2°C.
Ans. 134.3 h mol-1
For a mixture of two immiscible liquids, the total pressure is given by
...(i)
and...(ii)
Dividing equation (i) by (ii) gives,
or
where nl and nw represents the number of moles of liquid and water in vapour phase, respectively.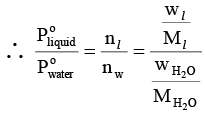
where Ml andrepresents molar mass of liquid and water respectively and wl and
denotes weight of liquid and water in vapour phase, respectively.
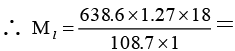
Q.8. 1000 gm of sucrose solution in water is cooled to –0.5°C. How much of ice would be separated out at this temperature, if the solution started to freeze at –0.38°C. Express your answer is gram. (Kf H2O = 1.86 K mol–1 kg ).
Ans. 0223 gm
wA + wB = 1000 …(i)
or
wB/wA = 0.07 …(ii)
By (i) and (ii), wB = 65.42 gm and wA = 934.58 gm
At –0.5°C, some water separated out as ice and solute exist as 65.42 gm.
wA = 711.58 gm
∴ separated ice = 934.58 gm – 711.58 gm = 0223 gm.
Q.9. What weight of solute (molecular weight 60) is required to dissolve in 180 g of water to reduce the vapour pressure to 4/5th pure water?
Ans. 150 g.
Ps = 4P°/5, m = 60, w = ?, W = 180 g, M = 18
Q.10. The freezing point of 0.08m NaHSO4 is –0.345°C. Calculate the percentage of that transfer a proton to water to form SO-24 ion.
Kf of H2O = 1.86 mol–1 kg.
Ans. 66%
NaHSO4 + H2O(aq) → Na+ + H3O+ + SO4–2
ΔTf = i × Kf × m
0.345 = (1 + 2α) × 1.86 × 0.08
So, α = 0.6592
% dissociation = 65.92%
|
481 docs|964 tests
|