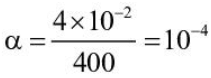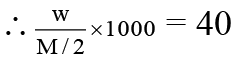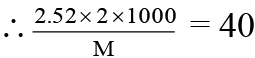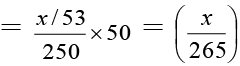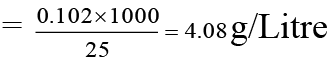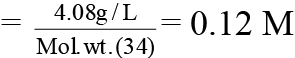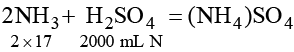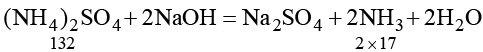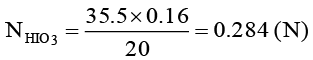Integer Answer Type Questions for JEE: Some Basic Concepts of Chemistry | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. Molar conductance of saturated BaSO4 solution is 400ohm−1cm2 mol−1 and its specific conductance is 4 × 10−5 ohm−1 cm−1. Hence solubility product value of BaSO4 can be concluded as x ×10−8 M2. Identify the x.
Ans. 1
Sp conduct = 4 x 10-5
A = 1000 x 4 x 10-5
= 4 x 10-2
∴
Ksp = 10-4 x 10-4 = 10-8 M2
Q.2. 10 ml of a gaseous hydrocarbon were burnt completely in 80 ml of O2 at N.T.P. The remaining gas occupied 70 ml at N.T.P. This volume becomes 50 ml on treatment with KOH solution. What is the empirical formula weight of hydrocarbon?
Ans. 14
Let the molecular formula of hydrocarbon be CxHy.
CxHy + (x + y/4)O2 → xCO2 + y/2 H2O.
Volume reacted 10 ml 10(x + y/4) - -
Volume produced 10x = 20 10x negligible
x = 2.
Since, the remaining gas occupied 70 ml,
∴ Total volume left = 70 = VO2 + VCO2
where VO2 = volume of oxygen left and VCO2 = Volume of CO2 produced.
∴ VO2 = 70 - 20 = 50 ml.
80 - 10(x + y/4) = 50
x + y/4 = 3
y/4 = 1
y = 4.
So, formula is C2H4.
Thus, empirical formula of the hydrocarbon is CH2 and its empirical formula weight is 14.
Q.3. A solution is containing 2.52 g litre-1 of a reductant. 25 ml of this solution required 20 ml of 0.01 M KMnO4 in acid medium for oxidation. Find the molecular weight of reductant. Given that each of the two atoms which undergo oxidation per molecule of reductant, show an increase in oxidation state by one unit.
Ans. 126
Meq. of reductant in 25 ml = Meq. of KMnO4
= 20 × 0.01 × 5
∴ Meq. of reductant in 1 litre = 20 × 0.01 × 5 × 40 = 40.
Reductant shows the change A2+a → 2A+b + 2e
∴ Equivalent weight of reductant = Molecular weight/2
∵ Meq. of reductant = 40
∴ M = 126.
Q.4. 1 gm of impure Na2CO3 is dissolved in water and solution made up to 250 ml. To 50 ml of this solution, 50 ml of 0.1 N HCl is added and mixture after shaking well, required 10 ml of 0.16 N NaOH solution for complete neutralization. Calculate % purity of sample of Na2CO3.
Ans. 90.1%
Let x gm be the weight of pure Na2CO3 in the sample.
Number of equivalents of Na2CO3 = x/53
Number of equivalents of Na2CO3 in 50 ml
Total meq. of HCl = 50 × 0.1 = 5
Meq. of HCl used by NaOH = 10 × 0.16 = 1.6
Meq. of HCl used by Na2CO3 = 5 - 1.6 = 3.4
∴ x/265 = 3.4 × 10-3 ; x = 0.901 g
% purity of Na2CO3 = 0.901/1 × 100 = 90.1%.
Q.5. To a 25 mL H2O2 solution, excess of acidified solution of potassium iodide was added. The iodine liberated required 20 mL of 0.3 N sodium thiosulphate solution. Calculate the volume strength of H2O2 solution. Express your answer in millilitre.
Ans. 1344 ml.
2KI + H2SO4 + H2O2 → K2SO4 +2H2O + I2
2Na2S2O3 + I2 → Na2S4O6 + 2NaI
Meq of Na2S2O3 = 20 × 0.3 = 6
Meq of Na2S2O3 ≡ Meq of I2 = 6
Meq of I2 ≡ Meq of H2O2 = 6
Wt. of H2O2 = Meq × Eq. wt. × 10–3 = 6 × 17 × 10–3 = 0.102 g
(∵ Eq. wt . of H2O2 = 34/2 = 17)
Strength of H2O2
Molarity of H2O2
2H2O2 → 2H2O + O2
2 Moles 1 Mole
∴ 0.12 Moles 0.06 Mole
Volume of O2 at STP = 0.06 × 22.4 Litre = 1.344 Litre
Hence, the volume strength of H2O2 = 1.344 Litre =1344 ml.
Q.6. 0.56 gm sample of lime stone is dissolved and all Ca is precipitated as CaC2O4. These required 40 mL of 0.25 N KMnO4 solution in acidic medium. Find the percentage of CaO in the lime stone.
Ans. 50%
Meq. of KMnO4 = 40 × 0.25 = 10
Meq. of CaO = 10
gm eq. of CaO = 10 × 10-3 = 1/100
Mass of CaO = 1/100 × 56/2 =0.28
% of CaO = 0.28/0.56 × 100 = 50%
Q.7. A definite amount of impure sample of P4O6 is treated with 20ml of X (M) KMnO4 in acidic medium to produce H3PO4 and MnCl2. 20 ml of same KMnO4 on treatment with 0.2 M FeSO4 requires exactly 10 ml of FeSO4 solution. What is the amount of pure P4O6. If 0.1 g sample is taken calculate % purity of P4O6.
Ans. 55%
Mn+7 + 5e → Mn2+ (acidic medium)
P4O6 + KMnO4 → H3PO4 + MnCl2
Meq of KMnO4 = Meq of P4O6
Meq of KMnO4 = 10 × 0.2 × 1
or, 20 × 5 × X = 10 × 0.2 × 1
or, X = 0.02 M
∴ W/220 × 8 × 1000 = 20 × 0.02 × 5
W = 0.055 gm
% purity of P4O6 = 55%.
Q.8. 1 gm of impure Na2CO3 is dissolved in water and the solution is made upto 250 ml. To 50 ml of this made up solution, 50 ml of 0.1 N HCl is added and the mixture after shaking well, required 10 ml of 0.16 N NaOH solution for complete neutralization. Calculate % purity of the sample of Na2CO3. (Express your answer to the nearest whole number).
Ans. 90%.
Meq of HCl = 50 × 0.1 = 5
Meq of NaOH = 10 × 0.16 = 1.6
Meq of HCl = Meq of Na2CO3 + Meq of NaOH
Meq of Na2CO3 in 50 ml solution = 5 – 1.6 = 3.4
∴ In 250 ml Meq of Na2CO3 = (3.4 × 250) / 50 = 17
W/106 × 2 × 1000 = 17
W = 0.901 gm
% purity = 0.901/1 × 100 = 90.1 ≅ 90%.
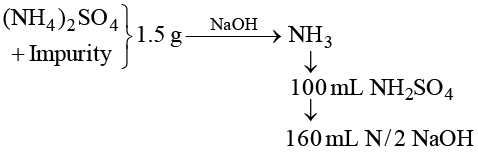
Q.9. 1.5 g of a sample of ammonium sulphate was boiled with excess of NaOH solution. Evolved NH3 was passed in 100 mL H2SO4. The partially neutralized acid required 160 mL N/2 NaOH for complete neutralization. Calculate % purity of ammonium sulphate ample.
Ans. 88
160 mL N/2 NaOH = 80 mL N NaOH ≡ 80 mL NH2SO4
∴ Consumed acid = 100 – 80 = 20 mL N H2SO4
2000 mL N H2SO4 ≡ 2 × 17 g NH3 ≡ 132 g (NH4)2SO4
∴ 20 mL NH2SO4 ≡ 132 × 20/2000 = 1.32 g (NH4)2SO4
% (NH4)2SO4 = 1.32/1.5 × 100 = 88.
Q.10. 20 ml solution of HIO3 is reacted with excess of an aqueous solution of SO2. The excess of SO2 and I2 formed are removed by heating the solution. The remaining solution in neutralized by 35.5 ml of 0.16 (N) NaOH solution. Calculate the strength of HIO3 in gm/lt.
(Round off the answer to the nearest whole number)
Ans. 10 gm/lt.
20 × NHIO3 = 35.5 × 0.16
N factor of HIO3 = 5
Mass of HIO3 = 0.284 × 176/5 = 10 gm/lt.
|
481 docs|964 tests
|