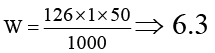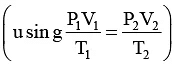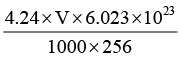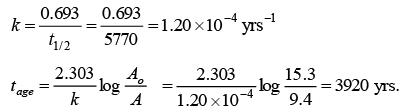Integer Answer Type Questions for JEE: Surface Chemistry | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. 50 ml of 1 M oxalic acid is shaken with 0.5 gm of wood charcoal. The final concentration of the solution after adsorption is 0.5 M. Amount of oxalic acid absorbed per gm of charcoal is
Ans. 6.3
(Molecular weight of oxalic acid ⇒ 163)⇒ 6.3 gm.
Q.2. On addition of one ml solution of 10%NaCl to 10 ml gold sol in the presence of 0.25 gm of starch, the coagulation is just prevented. Starch has the following gold number.
Ans. 250
By definition gold number of starch is the amount of starch in mg added to 10 ml standard gold sol which prevents the coagulation of gold on adding 1 ml of 10% NaCl solution. So the amount of starch is 0.25g = 250 mg. Hence gold number is 250.
Q.3. The coagulation of 100 cm3 of gold solution is completely prevented by addition of 0.25 g of starch to it before adding 10 ml of 10% NaCl solution. The gold number of starch is
Ans. 25
Gold number of starch is the milligrams of starch added to 10 ml of standard gold sol just to prevent coagulation of Au sol when 1 mL of 10% NaCl is added to it.
10 mL of 10% NaCl is added to 100 mL of gold sol. In other words 1 mL of 10% aCl is added to 10 mL of gold sol.
Quantity of starch added to 10 mL of gold sol = 25 mg
∴ Gold number of starch = 25.
Q.4. For the coagulation of 115 mL of Sb2S3 sol, 10 mL of 1 M NaCl is required. The flocculation value of NaCl is
Ans. 80
Millimoles of NaCl added = 10
Volume of the sol containing NaCl solution = 115 + 10 = 125 mL.Flocculation value is the amount of electrolyte (in millimoles) that must be added to 1 L of the colloidal solution containing NaCl solution so as to bring about complete coagulation..
∴ Flocculation value of NaCl = (10x1000)/125 = 80 m mol L-1.
Q.5. A sample of charcoal weighing 6 g was brought into contact with a gas contained in a vessel of one litre capacity at 27°C. The pressure of the gas was found to fall from 700 to 400 mm of Hg. Calculate the volume of the gas (reduced to STP) that is adsorbed per gram of the adsorbent under the condition of the experiment (density of charcoal sample is 1.5 g cm-3).
Ans. 60.19
The adsorption is taking place in a closed vessel, thus when pressure falls there is correspondingly increase in volume and to keep volume constant, excess of the volume of the gas would be adsorbed.UsingP1V1 = P2V2
V2 == 1750 ml
Actual volume of the flask = 1000 - volume of charcoal
= 1000 - 6/1.50 = 996 ml
Volume of the gas adsorbed = 1750 - 996 = 754 ml
Volume of the gas adsorbed per gram of charcoal = 754/6 = 125.67 ml g-1
Volume of the gas adsorbed per gram at STP60.19 ml g-1.
Q.6. A solution of palmitic acid (M = 256 g mol-1) in benzene contains 4.24 g of acid per dm3. When this solution is dropped on a water surface the benzene evaporates and the palmitic acid forms a monomolecular film of the solid type. If we wish to cover an area of 500 cm2 with a monolayer, what volume of solution should be used? The area covered by one palmitic acid molecule may be taken to be 0.21 nm2.
Ans. 0.024
Let the volume of palmitic acid solution required to cover the desired area of 500 cm2 be V ml.
∴ Number of molecules of palmitic acid in V ml =
= 9.976 x 1018 x VThe area covered by these molecules = 9.976 x 1018 x V x 0.21 x 10-14 cm2
= 2.095 x 104 x V cm2
But this area is equal to 500 cm2
∴ 2.095 x 104 x V = 500
or V = 0.024 ml.
Q.7. The density of gold is 19 g / cm3. If 1.9 x 10-4 g of gold is dispersed in one litre of water to give a sol having spherical gold particles of radius 10 nm, then the number of gold particles per mm3 of the sol will be.
Ans. 2380000
Volume of the gold dispersed in one litre water Mass/DensityRadius of gold sol particle = 10 nm = 10x10-9 m = 10x10-7 cm = 10-6 cm
Volume of the gold sol particle == 4.19 x 10-18 cm3
No. of gold sol particle in 1x10-5cm3 == 2.38 x 1012
No. of gold sol particle in one mm3 == 2.38 x 106 = 2380000
Q.8. On analysis an ore of uranium was found to contain 0.277 g of 206Pb and 1.667 g of 238U. The t1/2 of 238U is 4.5 x 109 years. If all the lead were assumed to have come from the decay of 238U, what is the weight of 238U, which has changed to 206Pb and what is the age of the ore?
Ans. 0.319
206 g of lead have come from 238 g of uranium.
0.277 g of lead would have come from= 0.319 g of U.
Q.9. The beta activity of 1g of carbon made from green wood is 15.3 count per minute. If the activity of 1g of carbon derived from the wood of Egyption mummy case is 9.4 counts per minute under the same condition, how old is the wood of the mummy case?
[t1/2 for 14C = 5770 hears)
Ans. 3920
In geological dating: (238U - 206Pb method)
Q.10. How many α and β- particles are emitted by 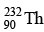 to reach
to reach 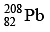 ?
?
Ans. 4
The reaction is, therefore, 232 = 208 + 4x ⇒ x = 6 , hence α- particles emitted = 6.
also, 90 = 82 + 2x - y ⇒ y = 4 , hence β- - particles emitted = 4.
|
446 docs|929 tests
|