Ionization of Polybasic Acids | Chemistry for JEE Main & Advanced PDF Download
Dissociation constants of acids and Bases
(1) Dissociation constant for weak acid: Consider an acid HA which, when dissolved in water ionizes as,
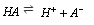
Applying the law of mass action, 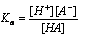
Where,  is the dissociation constant of the acid,
is the dissociation constant of the acid,  . It has constant value at definite temperature and does not change with the change of concentration.
. It has constant value at definite temperature and does not change with the change of concentration.
Dissociation Constant for polybasic acid: Polybasic acids ionise stepwise as, for example, orthophosphoric acid ionises in three steps and each step has its own ionisation constant.
 ⇌
⇌  (I step)
(I step)
 ⇌
⇌  (II step)
(II step)
 ⇌
⇌  (III step)
(III step)
Let  and
and  be the ionization constants of first, second and third steps respectively. Thus,
be the ionization constants of first, second and third steps respectively. Thus,
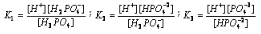
In general, 
The overall dissociation constant is given by the relation,
is given by the relation,

(2) Dissociation constant for weak base: The equilibrium of  (a weak base) can be represented as,
(a weak base) can be represented as,
 ⇌
⇌ 
Applying the law of mass action,
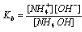
 is constant at a definite temperature and does not change with the change of concentration.
is constant at a definite temperature and does not change with the change of concentration.
|
334 videos|680 docs|300 tests
|
FAQs on Ionization of Polybasic Acids - Chemistry for JEE Main & Advanced
| 1. What is the definition of ionization of polybasic acids? |  |
| 2. How does the ionization of polybasic acids differ from monobasic acids? |  |
| 3. What factors affect the degree of ionization of polybasic acids? |  |
| 4. Can you provide an example of a polybasic acid and its ionization reaction? |  |
| 5. How does the degree of ionization of polybasic acids affect their acidity? |  |
















