JEE Advanced (Fill in the Blanks): Electrochemistry | Chapter-wise Tests for JEE Main & Advanced PDF Download
Fill in the Blanks
1. Of the halide ions, _____ is the most powerful reducing agent. (1978)
Ans: I–
Solution: ( ∵ I2 is weakest oxidising agent)
2. The more ............... the standard reduction potential, the ............... is its ability to displace hydrogen from acids. (1986 - 1 Mark)
Ans: negative, greater
Solution: negative, greater; Among the various metals, since sodium has the minimum reduction potential, it must be strongest reducing agent. In general, more the reduction potential
lesser is its reducing action.
3. The electrical conductivity of a solution of acetic acid will be .............. if a solution of sodium hydroxide is added. (1987 - 1 Mark)
Ans: increased
True/False
The dependence of electrode potential for the electrode Mn+ /M with concentration under STP conditions is given by the expression :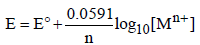 (1993 - 1 Mark)
(1993 - 1 Mark)
Ans: False
Solution : False : When the temperature is 273, the value of the factor will come out as 0.0541 instead of 0.0591. The value 0.0591 comes out at 298 K and not at 273 K.
|
446 docs|929 tests
|
FAQs on JEE Advanced (Fill in the Blanks): Electrochemistry - Chapter-wise Tests for JEE Main & Advanced
| 1. What is electrochemistry? |  |
| 2. What is the importance of electrochemistry? |  |
| 3. What is the Nernst equation? |  |
| 4. How does a galvanic cell work? |  |
| 5. What is Faraday's law of electrolysis? |  |
















