JEE Advanced (Fill in the Blanks): The p-Block Elements | Chapter-wise Tests for JEE Main & Advanced PDF Download
Fill in the Blanks
Q.1. The lowest possible oxidation state of nitrogen is (1980)
Ans. –3
Solution. –3;
Q.2. Iodine reacts with hot NaOH solution. The products are NaI and ....... (1980)
Ans. NaIO3
Solution. NaIO3
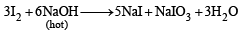
Q.3. ................. is a weak acid. (HF, HCl, HI) (1981 - 1 Mark)
Ans. HF
Solution. HF; HF is the weakest of the three, because the ionisation (i.e. acidic character) of HX is a multistep process and when its DH, heat of ionisation, is calculated it comes out to be the minimum. This is due to the strong H – F bond, large heat of hydration (because of H-bonding) and low value of electron affinity of F-atom.
Q.4. The increase in the solubility of iodine in an aqueous solution of potassium iodide is due to the formation of ............. . (1982 - 1 Mark)
Ans. KI3
Solution. KI3; complexes are more soluble in water as compared to normal salts. [KI+I2 → KI3]
Q.5. Hydrogen gas is liberated by the action of aluminium with concentrated solution of .............. . (1987 - 1 Mark)
Ans. sodium hydroxide
Solution. sodium hydroxide;
Al + 2NaOH + 2H2O → 2NaAlO2 +3H2
Q.6. .............. phosphorus is reactive because of its highly strained tetrahedral structure. (1987 - 1 Mark)
Ans. white/ yellow
Solution. white/ yellow; NOTE : In white phosphorus, each phosphorus atom is linked to the other three atoms by covelent bonds. PPP bond angle is 60°, due to which the molecule remains under strain and hence is active in nature.
Q.7. ............... acid gives hypo ............... ion. (1988 - 1 Mark)
(hydrobromic, hypobromous, perbromic, bromide, bromite, perbromate)
Ans. hypobromous, bromide
Solution. Hypobromous; bromite. 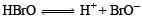
Q.8. Sulphur acts as ............... agent in vulcanization of rubber. (1989 - 1 Mark)
Ans. cross -linking
Solution. cross-linking;
Q.9. The basicity of phosphorous acid (H3PO3) is ............... . (1990 - 1 Mark)
Ans. two
Solution. 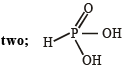 [It contains two replaceable hydrogens.]
[It contains two replaceable hydrogens.]
Q.10. The hydrolysis of alkyl substituted chlorosilan es gives .............. .
Ans. silicones
Solution. Silicones;
Q.11. In P4O10, the number of oxygen atoms bonded to each phosphorus atom is ............... . (1992 - 1 Mark)
Ans. 4
Solution. four. 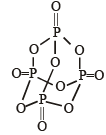
In each P atom is linked to 4 oxygen atoms
Q.12. The lead chamber process involves oxidation of SO2 by atomic oxygen under the influence of ............... as catalyst. (1992 - 1 Mark)
Ans. Nitric oxide
Solution. Nitric oxide. [NO]
The mixture containing SO2, air and nitric oxide, when treated with steam, sulphuric acid is formed.
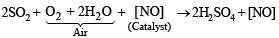
Q.13. The hydrolysis of trialkylchlorosilane R3SiCl, yields ........... (1994 - 1 Mark)
Ans. trialkylchlorosilanol
Solution. Trialkylchlorosilanol; The hydrolysis of R3SiCl, yields R3Si(OH) which condenses to give R3 Si – O – SiR3
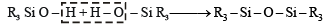
Q.14. One recently discovered allotrope of carbon (e.g., C60) is commonly known as........... (1994 - 1 Mark)
Ans. fullerene
Solution. Fullerene
Q.15. Solubility of iodine in water is greatly increased by the addition of iodide ions because of the formation of ........ (1994 - 1 Mark)
Ans. I–3 complex ion
Solution. I–3 complex ion I2 + I– ——→ I3-
Q.16. A liquid which is permanently supercooled is frequently called a ........... . (1997 - 1 Mark)
Ans. glass
Solution. glass
Q.17. Compounds that formally contain Pb4+ are easily reduced to Pb2+. The stability of the lower oxidation state is due to ........... . (1997 - 1 Mark)
Ans. inert-pair effect
Solution. inert-pair effect ; When ns2 electrons of outermost shell do not participate in bonding it is called inert pair and the effect is called inert pair effect.
|
446 docs|929 tests
|





















