JEE Advanced (Matrix Match & Integer Answer): General Principles & Processes of Isolation of Element | Chapter-wise Tests for JEE Main & Advanced PDF Download
Direction : question contains statements given in two columns, which have to be matched. The statements in Column-I are labelled A, B, C and D, while the statements in Column-II are labelled p, q, r, s and t.
Q.
Match the extraction processes listed in Column I with metals listed in Column II : (2006 - 6M)
Ans: (A)- p, r; (B) - p, r; (C) - q; (D) - s
| Column I | Column II |
| (A) Self reduction | (p) Lead |
| (B) Carbon reduction | (q) Silver |
| (C) Complex formation and displacement by metal | (r) Copper |
| (D) Decomposition of iodide | (s) Boron |
Solution :
NOTE : The oxides and sulphides of less active metals like Hg, Cu & Pb are unstable to heat and hence no reducing agent is required. They undergo self reduction.
(A) 2Cu2S + 3O2 →2Cu2O+ 2SO2 ;
Cu2S + 2Cu2O →6Cu + SO2
(B) 2PbS + 3O2 → 2PbO + 2SO2 ;
PbS + 2PbO → 3Pb + SO2
Hence (A) → (p), (r)
NOTE : The oxides of less electropositive metals like Pb, Zn, Fe, Sn, Cu, etc. are reduced by strongly heating them with coke or coal.
PbO + C → Pb + CO2 ;
2Cu2O+ C → 4Cu + CO2
Hence (B) → (p), (r)
Extraction from argentite (Ag2S)
Ag2S + 2NaCN  Na2S + 2AgCN;
Na2S + 2AgCN;
Sod. argentocyanide soluble
AgCN+ NaCN → Na[Ag(CN)2 ]
Zn, being more electropositive than Ag, displaces Ag from the complex.
2Na[Ag(CN)2 ] + Zn → Na2[Zn(CN)4 ] + 2Ag¯
Hence (C) →(q)
Among the halides of boron, BI3 is unstable because of the large size of Iodine and small size of boron atom. Hence it decomposes to give boron. Thus, (D) → (s).
2. Match the conversions in Column I with the type(s) of reaction(s) given in Column II. (2008 - 6M)
| Column I | Column II |
| (A) PbS → PbO | (p) roasting |
| (B) CaCO3 → CaO | (q) calcination |
| (C) ZnS → Zn | (r) carbon reduction |
| (D) Cu2S → Cu | (s) self reduction |
Ans : (A) - p; (B) - q; (C) - p, r; (D) - p, s
3. Match the anionic species given in Column-I that are present in the ore(s) given in Column-II. (JEE Adv. 2015)
| Column I | Column II |
| (A) Carbonate | (p) Siderite |
| (B) Sulphide | (q) Malachite |
| (C) Hydroxide | (r) Bauxite |
| (D) Oxide | (s) Calamine |
| (t) Argentite |
Ans: (A)- p,q,s, (B)- t, (C)- q, r, (D)- r
Solution :

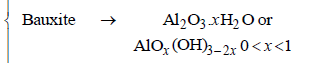

|
481 docs|964 tests
|





















