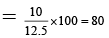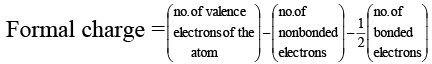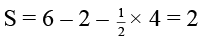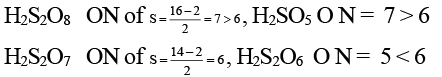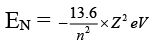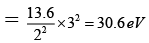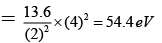JEE Advanced (One or More Correct Option): Chemical Bonding & Molecular Structure | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. The statements that are true among the followings are
(a) The bond order in CO molecule is 3
(b) The order of stability is 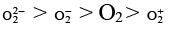
(c) KHF2, KHCl2 and KHBr2 are known but KHI2 is not
(d) NO is paramagnetic while NO+ is diamagnetic
Correct Answer is option (a, d)
Bond order O2–2 = 1
O2– = 1.5
O2 = 2
O2+ = 2.5
∴ stability; O2+ > O2 > O2– > O2–2
Q.2. KCl has a dipole moment of 10 D. The inter ionic distance in KCl is 2.6 Å. Which of the following statements are true for this compound?
(a) The theoretical value of dipole moment, if the compound were completely ionic is 12.5 D.
(b) The % ionic character of the compound is 85 %.
(c) It is a poor conductor of electricity.
(d) The forces operating in this molecule are coulombic type.
Correct Answer is option (a, c, d)
μ = q × d = 4. 8 ×10–10 × 2.6 × 10–8 esu unit
= 12.48 D ~ 12.5 D
(In case of 100% ionic character)
% Ionic character
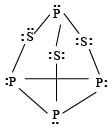
Q.3. Which of the following statements is/are true for P4S3 molecule?
(a) It contains six P-S bonds and three P-P-bonds
(b) It contains six P-S bonds and ten lone pairs
(c) It has all atoms sp3–hybridised
(d) It contains six P-P bonds and ten lone pairs
Correct Answer is option (a, b, c)
Q.4. Which of the following is/are correct?
(a) Ammonium salts are more soluble than sodium salts
(b) SnCl2 is more ionic than SnCl4
(c) Calcium fluoride is more ionic than calcium chloride.
(d) The formal charge on S atom in SO2 is four
Correct Answer is option (a, b, c)
Because of the H–bondingis more extensively solvated than Na+ , hence
salts is more soluble than Na+ salts.
The polarising power of Sn4+ is greater than Sn2+ , hence SnCl4 is more covalent The polarisibility of Cl– is greater than F– because of greater size.
In SO2 , formal charge of
Q.5. Which of the following order of solubilities is/are correct?
(a) LiF < LiCl < LiBr < LiI
(b) LiF < NaF < KF < RbF
(c) CsI < CsBr < CsCl < CsF
(d) CsI < KI < NaI < LiI
Correct Answer is option (a, b, c, d)
If the size of common ion is smaller, then greater the size of other ion more will be the solubility. If the size of common ion is bigger, then smaller the size of other ion more will be the solubility.
Q.6. Which of the following molecules has as O—O bond?
(a) H2S2O8
(b) H2S2O7
(c) H2SO5
(d) H2S2O6
Correct Answer is option (a, c)
Q.7. Which of the following species is paramagnetic?
(a) CN–
(b) NO
(c) O22-
(d) O2
Correct Answer is option (b, d)
CN– isoelectronic with N2, diamagnetic,
O2–2 isoelectronic with F2, diamagnetic ,
O2 → 2e– unpaired in π* orbital
NO → 15 electron paramagnetic
Q.8. Correct statements are:
(a) I.E. for hydrogen atom = 13.6 ev
(b) I.E. of Li2+ in the first excited state is 30.6eV
(c) I.E. of Be3+ in the first excited state is 54.4 eV
(d) I.E. of Li2+ in the first excited state in 10.6 eV
Correct Answer is option (a, b, c)
(a) E1 for H = -13.6 eV
(b) I.E. for LI+2 in 1ST exited state
(c) I.E of BE+3 in 1ST excited state
Q.9. Which of the molecules is/are planar?
(a) F2C = C= CF2
(b) F2B - C= C - BF2
(c) (SiH3)3N
(d) H2N - NH2
Correct Answer is option (a, b, c)
In F2C = C= CF2
Terminal C-atoms are sp2 hybridised, one sp2 hybridised orbital comes in the horizontal plane and other sp2 hybridised orbital appears on the vertical plane.
Q.10. Which of the following is correct?
(a) Ionic bond is non directional
(b) Ionic compound has very high boiling and melting point
(c) Ionic bond is form by transfer of electrons from one atom to another atom
(d) Boiling point of ionic compound is very low
Correct Answer is option (a, b, c)
Boiling point of ionic compound is very high because oppositely charged ions are healed together by very strong electrostatic attraction forces.
|
481 docs|964 tests
|