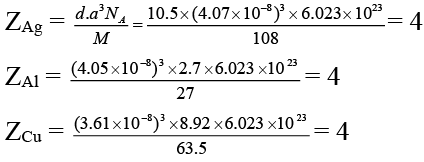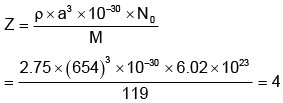JEE Advanced (One or More Correct Option): States of Matter | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. Which of the following are not true about hexagonal close packing?
(a) It has a coordination number of 6.
(b) It has 26% empty space.
(c) It is ABCABC…. type of arrangement.
(d) It is as closely packed as body centred cubic packing.
Correct Answer is option (a, c and d)
Hexagonal close packing has coordination number of 12. It is ABAB type of arrangement and is more efficient than body centred packing.
Q.2. The data given below is used to find the type of cubic lattice to which the crystal belong.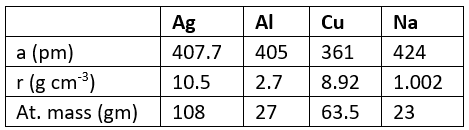
Which of the above crystallizes in FCC unit cell
(a) Ag
(b) Al
(c) Cu
(d) Na
Correct Answer is option (a, b and c)
Hence all crystallizes in FCC unit cell.
Q.3. Which of the following is/are correct for Cs Cl type crystal:
(a) It is ideal BCC structure.
(b) Cs+ and Cl- both ions forms a simple cubic lattice which inter penetrate each other.
(c) Cs+ ions are at the body centre of Cl- unit cell
(d) Cl- ions are at the body centre of Cs+ unit cell.
Correct Answer is option (b, c and d)
CsCl is BCC-like structure. It is form by interpenetration of the two primitive array of Cs+ ion & Cl- ion.
Q.4. A salt MX crystallising in KCl type of structure in which K+ is forming fcc.
(a) The nearest neighbour of Cl– ion is 6 K+ ion
(b) The packing fraction is given by 1.732π/8
(c) The nearest neighbour of K+ ion is 6 Cl– ion
(d) The second nearest neighbour of K+ ion is 12 K+ ion.
Correct Answer is option (a, c and d)
KCl crystallise in rock salt having six nearest neighbor of K+ and Cl– ion. Packing fraction is 0.74. The number of 2nd nearest neighbor of cation is 12.
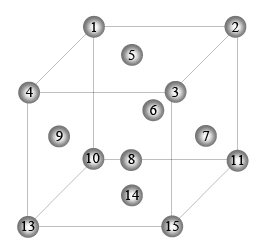
Q.5. A layered sequence for an FCC = CCP metal is shown below: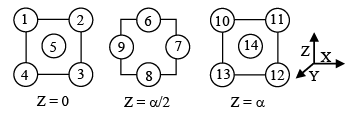
A face diagonal passes through the centre of atom 4 and the centre (s) of which other atom (s)?
(a) 1
(b) 2, 5
(c) 8, 12
(d) 9, 10
Correct Answer is option (b, c and d)
Q.6. Select the correct statement(s)
(a) For CsCl unit cell (edge-length = a), rc + ra = √3/2a
(b) For NaCl unit cell (edge-length = λ), rc + ra = ℓ/2
(c) The void fraction in a b.c.c. unit cell is 0.68
(d) The void space % in a face-centered unit cell is 26%
Correct Answer is option (a, b and d)
In bcc structure are r+ + r– = √3/2a
Hence, for CsCl, rc + ra = √3/2a
Since, NaCl crystallise in fcc structure
∴ 2rc + 2ra = edge length of the unit cell
Hence, rc + ra = I/2
Since packing fraction of a bcc unit cell is 0.68
∴ void space = 1–0.68 = 0.32
In fcc unit cell, packing percentage = 74%
∴ void percentage = 100–74 = 26%
∴ (A), (B) & (D)
Q.7. Select the correct statements:-
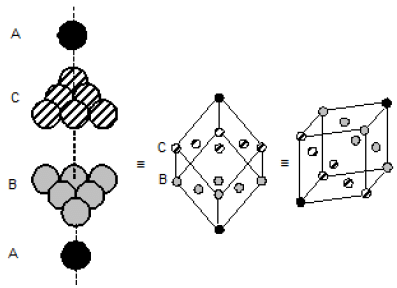
(a) In closest packing of x-atoms, there are 2x tetrahedral holes, & x-octahedral holes.
(b) The co-ordination no. of U:O in UO2 is 8:4
(c) In hexagonal packing of oxide ion Sapphire (the aluminium oxide) Al+3 ions crystallize in 2/3rd of the Octahedral hole, then the formula of Sapphire is Al2O3
(d) If anion B constitutes ccp and all the octahedral voids are occupied by cation (A), the crystal has the same molecular formula AB, if the cation (A) occupies the alternate tetrahedral voids.
Correct Answer is option (a, b, c and d)
In a closest packing number of tetrahedral holes is twice of number of octahedral voids.
∴ A and B are correct
For an oxide ion there is one octahedral hole whose 2/3rd part is occupied by Al3+ ions.
∴ Number of Al3+ ions per unit cell = 1 × 2/3 = 2/3
Hence, the formula of the compound is Al2/3O1 or Al2O3
∴ (C) is correct
In ccp structure there are 8 tetrahedral holes and 4 octahedral holes
Anion B constitutes ccp
∴ Total number of B atoms = 4
Cations (A) occupy all the octahedral voids
∴ Total no. of A atoms = 4
So, A : B = 4 : 4
∴ Formula of the compound is AB
If cation occupies alternate tetrahedral voids
Total no. of cations = 4
Total no. of anions = 4
Again A : B = 4 : 4
∴ Formula of the compound is AB
∴ (D) is correct
Hence, (A), (B), (C) and (D) are the correct answers.
Q.8. Graphite is
(a) good conductor
(b) sp2 hybridized
(c) an amorphous solid
(d) a covalent crystal
Correct Answer is option (a, b and d)
Graphite is a good conductor, sp2 hybridized and a covalent crystal and crystalline (and not amorphous).
Hence, (A), (B) and (D) are the correct answers.
Q.9. The density of KBr is 2.75 g/cm-3. The length of the unit cell is 654 pm. Atomic mass of K = 39, Br = 80. Then what is true about the predicted nature of the solid?
(a) Unit cell is fcc
(b) Z = 4
(c) There are four constituents/unit cells
(d) There are 8 ions at corners and 6 at the centres of the faces
Correct Answer is option (a, b, c and d)
As Z = 4, it is fcc unit cell. There are four KBr units.
There are 8 ions at the corners and 6 at the face centres.
Hence (A), (B), (C) and (D) are the correct answers.
Q.10. The coordination number of FCC structure for metals is 12, since
(a) Each atom touches 4 other in same layer, 3 in layer above and in layer below.
(b) Each atom touches 4 other in same layer, 4 in layer above and 4 in layer below.
(c) Each atom touches 6 others in same layer, 3 in layer above and 3 in layer below.
(d) Each atom touches 3 others in same layer, 6 in layer above and 6 in layer below.
Correct Answer is option (b and c)
An atom present at face centre touches face-centre atoms of adjacent face centres and corner atoms of the face at which it is present.
If a face is considered as a layer then the face centre atom of that face will touch 4 atoms on that face (or layers), 4 atoms of above layer (consists of face centre atoms of one unit cell) and 4 atoms of below layer (consists of face centre atoms of adjacent unit cell).
(C) If we look FCC unit cell from cubic close packing arrangement view then, an atom touches 6 atoms in its layer, 3 atoms in above layer and 3 atoms in layer below it.
|
446 docs|929 tests
|