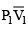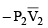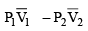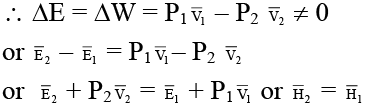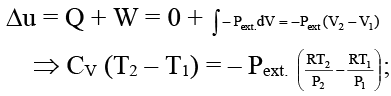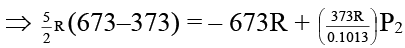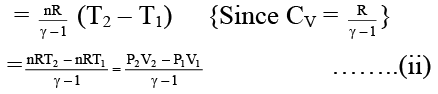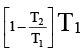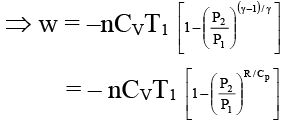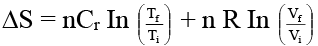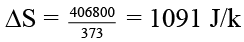JEE Advanced (One or More Correct Option): Thermodynamics | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. Enthalpy change for a reaction depends upon
(a) physical state of reactants and products
(b) initial and final concentration
(c) number of steps in the reaction
(d) conditions of reaction
Correct Answer is option (a, b and d)
(A), (B) and (D) explain: The conditions on which DH of a reaction depends are
(i) Physical state of reactants and products
(ii) Initial and final concentration
(iii) Conditions in which reaction is occurred
Hence choices (A), (B), (D) are correct while (C) is incorrect.
Q.2. Two bulbs R and S, connected by a mercury manometer, are held in a thermostat, as shown below. The volume of R is twice that of S. R contains as gas X at the same pressure as the nitrogen in S.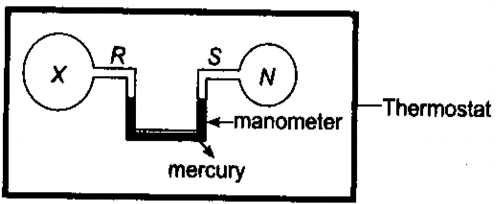
When the thermostat temperature is increased. Which of the following gases in bulb R would cause the mercury level in the right hand limb of the manometer to rise?
(a) An equilibrium mixture N2 g + 3H2g ⇌ 2NH3 g ; ΔH<0
(b) An equilibrium mixture CH3NC g ⇌ CH3CN g ; ΔH<0
(c) An equilibrium mixture N2F4 g ⇌ 2NF2 g ; ΔH>0
(d) An equilibrium mixture Ag ⇌ 2B g; ΔH=0
Correct Answer is option (a and c)
At initial stage, R contains twice as much of gas particle as S. If this ratio is maintained at different temperatures, the pressure in R and S will change but they will still be equal to one another, i.e., the Hg level will not change. Increase in temperature shifts the reaction backward and in (a) and forward in (c) which results in increase in pressure thus rise in right hand manometer.
Q.3. Select the correct statement(s) regarding phosphoric acid.
Given that ∆fH°(H3PO4) = -1290 kJ mol-1, ∆fH°(H2PO4-) = -1302 kJ mol-1, S°(H3PO4) = 176 J mol-1K-1, S°(H2PO4-) = 89 J mol-1K-1.
(a) It has a pKa1 of 2.4
(b) pKa2 of phosphoric acid is less than pKa1.
(c) First dissociation of phosphoric acid is exothermic.
(d) First dissociation of phosphoric acid is entropy driven.
Correct Answer is option (a and c)
Thermodynamics
H3PO4(aq) H2PO4-(aq) + H+(aq)
∆rH°= ∆fH°(H2PO4-)+∆fH°(H+) -∆fH°(H3PO4) = -1302+0-(-1290) = -12 kJ mol-1
∆rS° = S°(H2PO4-)+S°(H+) -S°(H3PO4) = 89+0-176 = -87 JK-1mol-1
∆rG°= ∆rH°-T∆rS° = -12000 + 298 × 87 = 13926 J
2.303RTpKa1 = 13926 ⇒ pKa1 = 2.44
Q.4. The given system is completely thermally insulated. P2 < P1 and T2 < T1 consider molar volume at P1, T1 is 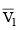 and molar volume at P2, T2 is
and molar volume at P2, T2 is 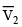 . 1 mole of gas is transferred from piston (I) to piston (II) As per the given figure -
. 1 mole of gas is transferred from piston (I) to piston (II) As per the given figure -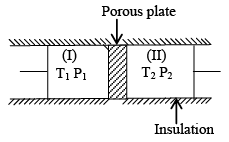
(a) work done on the gas in piston (I) is P1 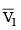 and work done by the gas in piston (II) is P2
and work done by the gas in piston (II) is P2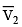
(b) Net work done, 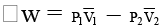
(c) The given process is constant enthalpic process.
(d) For the given process the change in internal energy, ΔE ≠ 0
Correct Answer is option (a, b, c and d)
Work done in piston (I) =
Work done in piston (II) =
ΔW =
Since system is completely thermally insulated, hence q = 0
Thus entire process is isoenthalpic process
Q.5. For the three elements P, Q & R, ionization enthalpy (IE) and electron gain enthalpies (Δeg H) are given in the following table -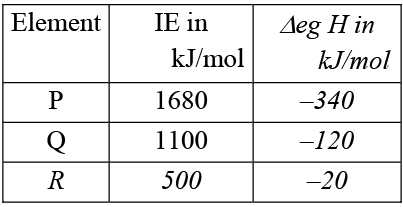
(a) P is the highest electronegative element among P, Q and R
(b) R is the least electronegative among P, Q & R
(c) Electro negativity of P is approximately equal to 4
(d) R may be chlorine
Correct Answer is option (a, b and c)
Greater the value of (IE – Δeg H) greater is the electronegativity E.N. of
P = (1680 + 340)/(4.18) (125) ≌ 4
Q.6. N2 gas is assumed to behave ideally. A given volume of N2 originally at 373 K and 0.1013 MPa pressure is adiabatically compressed due to which its temperature rises to 673 K. Which of the following statements is/are correct?
(a) The change in internal energy is 6235.5 J mol–1
(b) In this case the final internal pressure is equal to the external pressure
(c) The final pressure of N2 is approximately 0.38 MPa
(d) The final pressure of N2 is approximately 0.02 MPa
Correct Answer is option (a, b and c)
Here Pext = P2
⇒ P2 = 0.3865 M Pa
Δu = Cv (T2 - T1) J/mole
= 5/2 R × (673-373) J/mole
= 5/2 R × 300 = 5/2 × 9.314 × 300 J/mole
= 6235.50 J/mole
Q.7. The work involved in a reversible adiabatic process of expansion of an ideal gas from P1 and V1 to P2 and V2 is given by
(a) 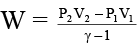
(b) 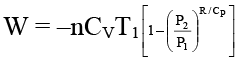
(c) W = nCv (T2 – T1)
(d) W = [efficiency of carnot cycle × aabsorbed]
Correct Answer is option (a, b and c)
Δu = Q + w ⇒ nCv ΔT = 0 + W (In adiabatic process Q = 0)
⇒ W = nCV (T2 – T1) ……..(i)
Again w = – nCV (T1 – T2) = –nCV
Q.8. One mole of an ideal diatomic gas (Cv = 5cal) was transformed from initial 25°C and 1L to the state when temperature is 100°C and volume 10L. Then for this process (R = 2 Calories/mol/K) (take calories as unit of energy and kelvin for temp)
(a) ΔH = 525
(b) ΔS = 5 ln 373/298 + 2 ln 10
(c) ΔE = 525
(d) ΔG of the process cannot be calculated using given information.
Correct Answer is option (a, b and d)
ΔH = nCv ΔT ⇒ ΔE = nCv ΔT
⇒ ΔG = ΔH - Δ (TS)
Q.9. 180 gm of water are evaporated slowly under isothermal conditions at 100ºC. Assuming water vapour to behave ideally (ΔHv = 2260 J/g) -
(a) ΔU = 375.8 kJ
(b) ΔS = 177.5 J/K
(c) ΔG = 0
(d) Q = 406.8 kJ
Correct Answer is option (a, c and d)
Qp = Qv + PΔV = Qv + P(Vv - Vλ)
= Qv + nRT (Θ Vv < < Vλ)
Qp = 2260 × 18 × 10 = 406.8 kJ
Qv = Qp – nRT = 406.8 – 10 × 8.314 × 10–3 × 373
= 375.8 kJ
Q.10. Enthalpy of atomisation of C2H6 (g) and C3H8 (g) are 620 kJ/mol and 880 kJ/mol. Therefore
(A) DC – H is 90 kJ/mol
(B) DC – C is 80 kJ/mol
(C) DC = C is 160 kJ/mol
(D) Enthalpy of atomisation of CH4 is approximately 360 kJ/mol.
Correct Answer is option (a, b and d)
DC–C + 6 DC – H = 620 kJ
2DC–C + 8 DC – H = 880 kJ
∴ 2DC–C + 12 DC – H = 1240
2DC–C + 8 DC – H = 880
∴ 4 DC – H = 360
DC – H = 90 kJ/mol
∴ DC - C = 620 - 6 × 90 = 620 - 540 = 80 kJ/mol
|
446 docs|929 tests
|