JEE Advanced (Subjective Type Questions): Chemical Bonding & Molecular Structure | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. Water is liquid while H2S is a gas at room temperature. (1978)
Ans. Sol. H2O molecules are held together by hydrogen bonding which is stronger force of attraction but H2S molecules are held together by vander waals forces of attraction, which are weaker forces. As a result water molecules come closer and exist in liquid state.
Q.2. Write the Lewis dot structural formula for each of the following. Give, also, the formula of a neutral molecule, which has the same geometry and the same arrangement of the bonding electrons as in each of the following. An example is
given below in the case of H3O+ :
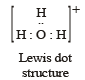
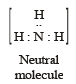
(i) O22- ; (ii) CO32- ; (iii) CN–; (iv) NCS– (1983 - 1 × 4 = 4 Marks)
Ans. Sol.
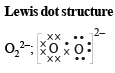

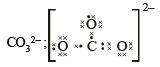
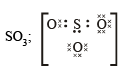
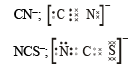
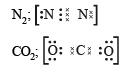
Q.3. How many sigma bonds and how many pi-bonds are present in a benzene molecule? (1985 - 1 Mark)
Ans. Sol.
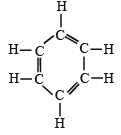 ∴ It has 12σ and 3π bonds.
∴ It has 12σ and 3π bonds.
(6σ C–C bonds and 6σ C–H bonds)
Q.4. Write the Lewis dot structure of the following : O3, COCl2
(1986 - 1 Mark)
Ans. Sol.
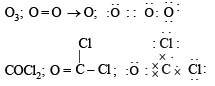
Q.5. Arrange the following : (i)N2, O2, F2, Cl2 in increasing order of bond dissociation en ergy. (1988 - 1 Mark)
(ii) Increasing strength of hydrogen bonding (X–H–X) : (1991 - 1 Mark)
O, S, F, Cl, N
(iii) In the decreasing order of the O – O bond length present in them (2004 - 4 Marks)
O2, KO2 and O2 [AsF4]
Ans. Sol. (i) Increasing order of bond dissociation energy.
F2 < Cl2 < O2 < N2
NOTE : Fluorine-fluorine bond energy is less than the Cl–Cl because of larger repulsion between the nonbonded electrons of the two smaller fluorine atoms (chlorine atoms are larger in size; hence their lone pair of electrons exert less repulsion than fluorine). Oxygen having two pairs of lone pair of electrons on each atom exert less repulsion than that of chlorine or fluorine each having three lone pairs of electrons. Nitrogen having only one lone pair of electrons exert minimum repulsion, hence it is the most stable.
(ii) H-bonding is an electrostatic attractive force between covalently bonded hydrogen atom of one molecule and an electronegative atom (F, O, N). Further, higher the electronegativity and smaller the size of the atom, the stronger is the hydrogen bond.
NOTE : Although Cl has the same electronegativity as nitrogen, it does not form effective hydrogen bonds.
This is because of its larger size than that of N with the result its electrostatic attractions are weak. Similarly, sulphur forms a very weak hydrogen bond due to its low electronegativity, although oxygen present in the same group forms a strong hydrogen bond.
Hence the order is S < Cl < N < O < F
(iii) In KO2, O2 is present as O2–, while in 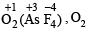
is present as O2+. Write down the MO configuration of O2, O2– and O2+.
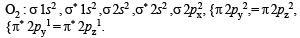
Thus the bond order =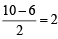
O2– : Same as above except 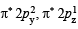 in place of
in place of 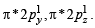
Thus the bond order in 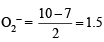
O2+ : Same as in O2 except 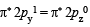 in place of
in place of
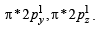
∴ Bond order in 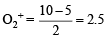
∴ Bond order in the three species is O2+ > O2 > O2– or O2[AsF4] > O2 > KO2
Q.6. The dipole moment of KCl is 3.336 × 10–29 Coulomb meters which indicates that it is a highly polar molecule. The interatomic distance between K+ and Cl– in this molecule is 2.6 ×10–10 m. Calculate the dipole moment of KCl molecule if there were opposite charges of one fundamental unit located at each nucleus. Calculate the percentage ionic character of KCl. (1993 - 2 Marks)
Ans. Sol. Dipole moment, µ = e × d coulombs metre For KCl d = 2.6 × 10–10 m
For complete separation of unit charge (electronic charge) (e) = 1.602 × 10–19 C
Hence µ = 1.602 × 10–19 × 2.6 × 10–10 = 4.1652 × 10–29 Cm µKCl = 3.336 × 10–29
Coulomb meter (given)
∴ % Ionic character of KCl =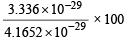
= 80.09%
Q.7. Using the VSEPR theory, identify the type of hybridization and draw the structure of OF2. What are the oxidation states of O and F ? (1994 - 3 Marks)
Ans. Sol. The structure of OF2 is similar to H2O and involves sp3 hybridization on O atom. The bond angle in F – O – F is not exactly 109º28’, but distorted (103º) due to presence of lone pair of electrons on O as well as F leading to V shape or tetrahedral positions with two positions occupied by lone pair of electrons of the molecule.
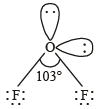
Oxidation number of F = –1
∴ Oxidation number of O = +2
Q. 8. A compound of vanadium has a magnetic moment of 1.73 BM. Work out the electronic configuration of the vanadium ion in the compound. (1997 - 2 Marks)
Ans. Sol. Magnetic moment (μ) = 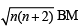
where n → number of unpaired electrons m = 1.73
BM for vanadium ion 1.73 BM =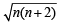 So, (1.73)2 = n(n + 2)
So, (1.73)2 = n(n + 2)
3.0 = n2 + 2n or n2 + 2n – 3 = 0
n2 + 3n – n – 3 = 0
∴ n(n + 3) – 1 (n + 3) = 0 (n – 1) (n + 3) = 0
Correct value of n = 1 Thus no. of unpaired electrons in vanadiumion = 1
23V = 1s2, 2s2 2p6, 3s2 3p6 3d3, 4s2
It will have one unpaired electron if it will lose two electrons from 4s and two from 3d.
∴ Vanadium (IV) has one unpaired electron.
V4+ = 1s2, 2s2 2p6, 3s2 3p6 3d1
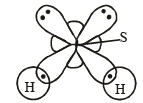
Q. 9. Interpret the non-linear shape of H2S molecule and nonplanar shape of PCl3 using valence shell electron pair repulsion (VSEPR) theory. (Atomic numbers : H = 1, P = 15, S = 16, Cl = 17.) (1998 - 4 Marks)
Ans. Sol. In H2S, no. of hybrid orbitals = 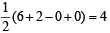
Hence here sulphur is sp3 hybridised, so
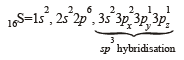
 or
or 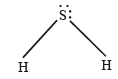
NOTE : Due to repulsion betwen lp - lp; the geometry of H2S is distorted from tetrahedral to V-shape.
In PCl3, no. of hybrid orbitals = 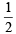 [5 + 3 - 0 + 0] = 4
[5 + 3 - 0 + 0] = 4
Hence, here P shows sp3 - hybridisation
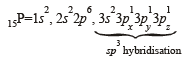
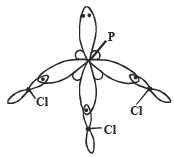
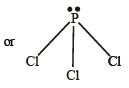
Thus due to repulsion between lp - bp, geometry is distorted from tetrahedral to pyramidal.
Q.10. Write the M.O. electron distribution of O2. Specify its bond order and magnetic property. (2000 - 3 Marks)
Ans. Sol. MO configuration of O2 :
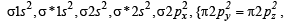
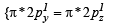
Bond order = 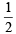 (10 - 6) = 2
(10 - 6) = 2
Since O2 molecule has two unpaired electrons, it is paramagnetic.
Q.11. Using VSEPR theory, draw the shape of PCl5 and BrF5. (2003 - 2 Marks)
Ans. Sol.

PCl5 : sp3d Trigonal bipyramidal
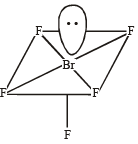
BrF5 : sp3d2 Square pyramidal
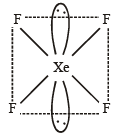
Q. 12. Draw the structure of XeF4 and OSF4 according to VSEPR theory, clearly indicating the state of hybridisation of the central atom and lone pair of electrons (if any) on the central atom. (2004 - 2 Marks)
Ans. Sol. First determine the total number of electron pairs around the central atom.
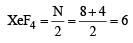
Thus in XeF4, Xe is sp3d2 hybridised. The structure of the molecule is octahedral and shape is square planer with two lone pair of electrons.
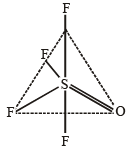
For OSF4 : 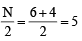
Thus the central atom (S) is sp3d hybridised leading to trigonal bipyramidal structure with no lone pair of electrons.
|
446 docs|930 tests
|






















