JEE Advanced (Subjective Type Questions): General Principles & Processes of Isolation of Elements | Chapter-wise Tests for JEE Main & Advanced PDF Download
1. (a) Write the chemical equations involved in the extraction of lead from galena by self reduction process.
(b) Match the following extraction processes with the appropriate metals listed below :
| (i) Silver | (A) Fused salt electrolysis |
| (ii) Calcium | (B) Carbon reduction |
| (iii) Zinc | (C) Carbon monoxide reduction |
| (iv) Iron | (D) Amalgamation |
| (v) Copper | (E) Self reduction |
(1979)
Solution :
(a) Galena is roasted in excess of air in a reverberatory furnace
2PbS + 3O2 → 2PbO + 2SO2
(air)
PbS + 2O2 → PbSO4
It is followed by self reduction
PbS + PbSO4 → 2Pb + 2SO2
PbS + 2PbO → 3Pb + SO2
(b) (i) Silver → (D) Amalgamation
(ii) Calcium → (A) Fused salt electrolysis
(iii) Zinc → (B) Carbon reduction
(iv) Iron → (C) Carbon monoxide reduction
(v) Copper → (E) Self reduction
2. Write the matching pairs:
| Bleaching agent | Aluminium |
| Smelling salt | Carbon |
| Cryolite | Tin |
| Bell metal | Ammonium carbonate |
| Fluorspar | Ammonium phosphate |
| Fertilizer | Calcium |
| Anthracite | Chlorine |
Examples :
Bleaching agent
Chlorine
Smelling salt
Ammonium carbonate
Solution :
Bleaching agent → Chlorine
Smelling salt → Ammonium carbonate
Cryolite → Aluminium
Bell metal → Tin
Fluorspar → Calcium
Fertilizer → Ammonium phosphate
Anthracite → Carbon
3. Give reasons for the following :
(i) Metals can be recovered from their ores by chemical methods. (1984 - 1 Mark)
(ii) High purity metals can be obtained by zone refining method. (1984 - 1 Mark)
(iii) Why is chalcocite roasted and not calcinated during recovery of copper? (1987 - 1 Mark)
solution :
(i) because they occur as oxides, carbonates, sulphides which have to be calcined or roasted.
(ii) NOTE : Zone refining is based on the difference in solubility of impurities in molten and solid state of the metal. This method is used for obtaining metals of very
high purity.
Ge, Si and Ga used as semi-conductors are refined in this manner. These metals can be easily melted and can easily crystallise out from the melt form.
(iii) Excess of Air (used during roasting) is necessary for converting chalcocite (a sulphide ore) to oxide. Calcination does not convert it to oxide.
4. Give the equations for the recovery of lead from Galena by air reduction. (1987 - 1 Mark)
Solution:
Recovery of Pb from galena :
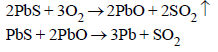
|
446 docs|929 tests
|
















